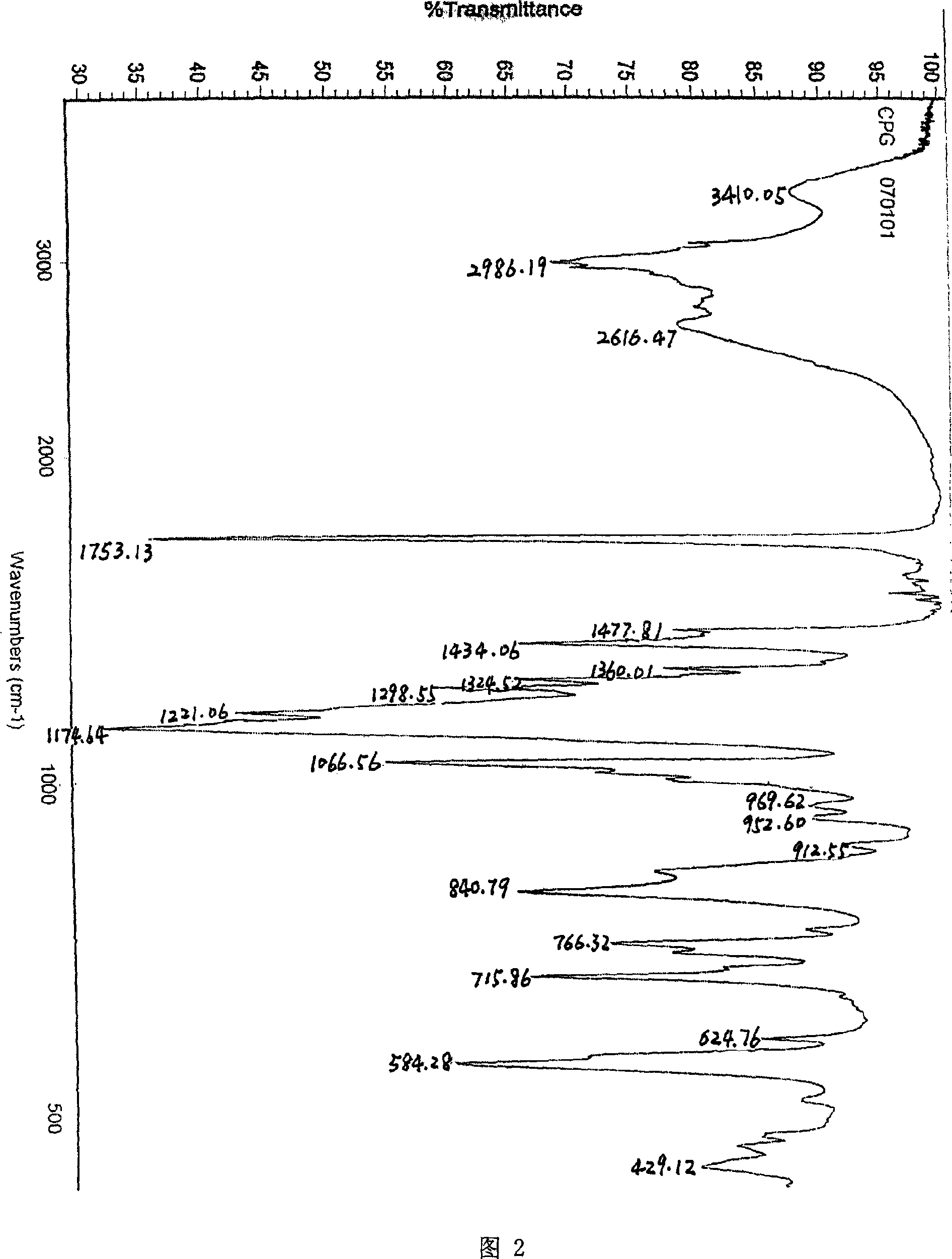
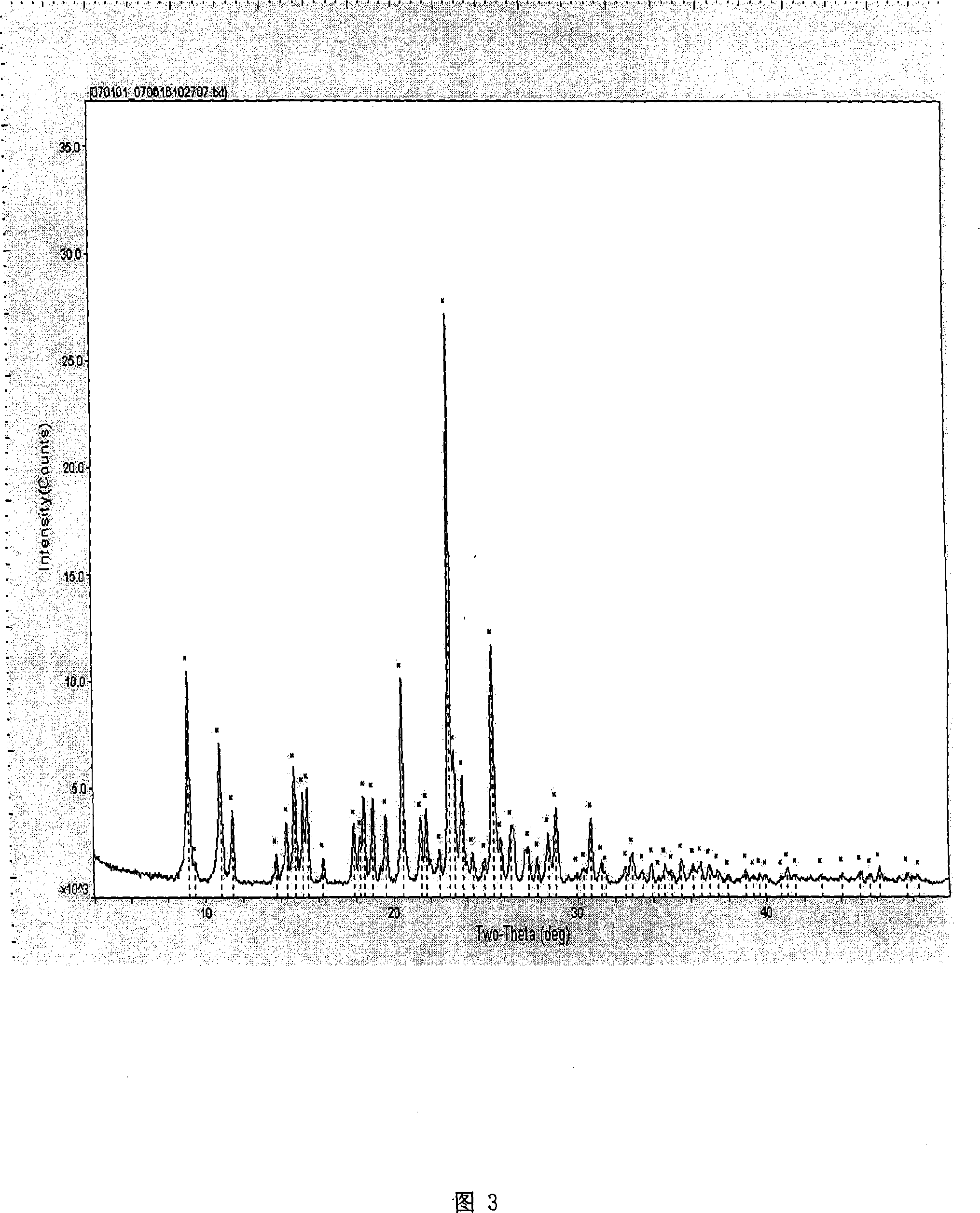
Method for preparing (+)-(S-)-clopidogrel hydrosulfate high melting point crystal I
A kind of technology of clopidogrel hydrogen sulfate and clopidogrel free base, which is applied in the field of preparation of high melting point crystal form I of clopidogrel hydrogen sulfate, and can solve the problems of harsh process conditions, easy crystal transformation, long storage time, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Put 5 g of clopidogrel free base and 35 ml of methyl ethyl ketone into a 100 ml three-necked flask, stir to dissolve and clarify, control the temperature at 25 ± 2 ° C, slowly add 1.5 g of concentrated sulfuric acid (mass fraction is 98%), after a small amount of crystals are precipitated, Add 0.5ml of methanol, stir and crystallize at this temperature for 12 hours. After the crystallization is completed, filter, wash with a small amount of butanone, and drain as much as possible to ensure that the residual amount of butanone in the filter cake is less than 10%. The filter cake was dried under vacuum at 30°C for 2-4 hours. 5.6 g of white powder solid was obtained, namely clopidogrel bisulfate crystal form I, with a yield of 86%. Melting point: 198-200°C.
Embodiment 2
[0020] Put 5g of clopidogrel free base and 35ml of acetone into a 100ml three-necked flask, stir to dissolve and clarify, control the temperature at 25±2°C, slowly add 1.5g of concentrated sulfuric acid (mass fraction is 98%) dropwise, after a small amount of crystals are precipitated, add Methanol 0.5ml, stirred and crystallized at this temperature for 12 hours, after the crystallization was completed, filtered, washed with a small amount of acetone, and drained as much as possible to ensure that the residual amount of acetone in the filter cake was less than 8%. The filter cake was dried under vacuum at 30°C for 2-4 hours. 5.5 g of white powder solid was obtained, which was the crystalline form I of clopidogrel bisulfate, and the yield in this step was 85%. Melting point: 198-200°C.
Embodiment 3
[0022] Put 5 g of clopidogrel free base and 35 ml of methyl ethyl ketone into a 100 ml three-necked flask, stir to dissolve and clarify, control the temperature at 25 ± 2 ° C, slowly add 1.5 g of concentrated sulfuric acid (mass fraction is 98%), after a small amount of crystals are precipitated, Add 0.5ml of absolute ethanol, stir and crystallize at this temperature for 12 hours, after the crystallization is completed, filter, wash with a small amount of butanone, and drain as much as possible to ensure that the residual amount of butanone in the filter cake is less than 10%. The filter cake was dried under vacuum at 30°C for 2-4 hours. 5.4 g of white powder solid was obtained, which was the crystalline form I of clopidogrel bisulfate, and the yield of this step was 83%. Melting point: 198-200°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com