Novel crystal form of ibrutinib and preparation method thereof
A technology of ibrutinib and crystal form, which is applied in the field of medicinal chemistry and can solve problems affecting the quality of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
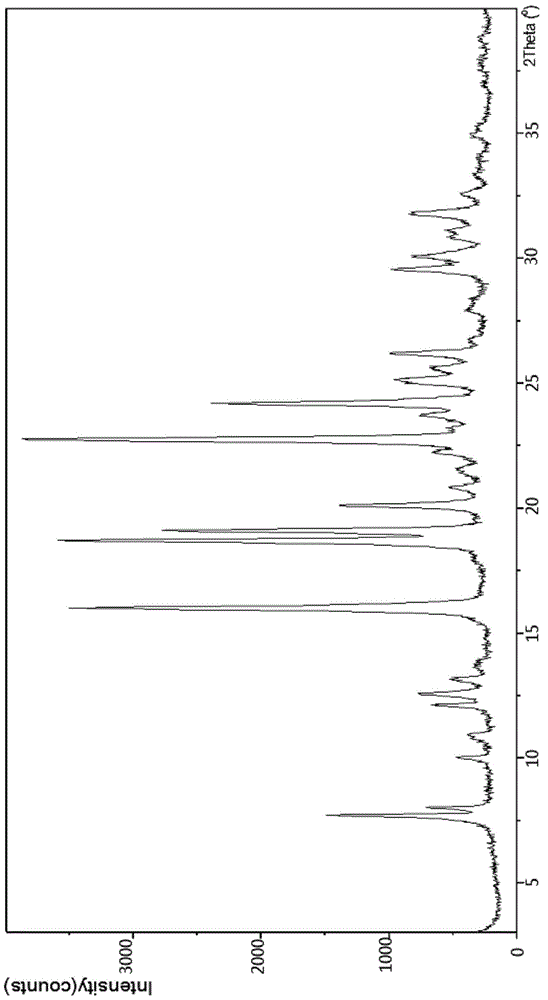
[0028] In the first aspect, the inventor developed a crystal form of ibrutinib through research, which is called crystal form A2.
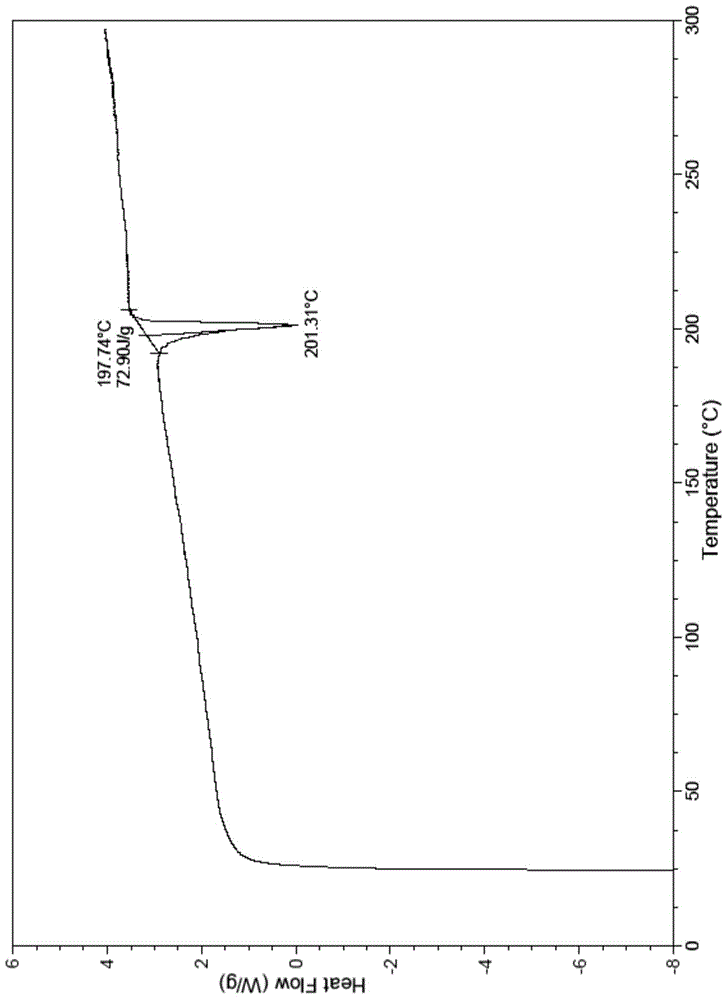
[0029] The crystal form A2 of ibrutinib has the following characteristics: its differential scanning calorimetry curve (DSC) has an endothermic peak at 185°C-210°C.
[0030] In some embodiments, the differential scanning calorimetry curve (DSC) of the crystalline form A2 of ibrutinib has an endothermic peak at 190°C-205°C. In some embodiments, the differential scanning calorimetry curve (DSC) of the crystalline form A2 of ibrutinib has an endothermic peak at 194°C-204°C. In some embodiments, the peak value of the endothermic peak of the differential scanning calorimetry curve (DSC) of the crystalline form A2 of ibrutinib is about 201.3°C. In some embodiments, the differential scanning calorimetry curve (DSC) of the crystalline form A2 of ibrutinib is as follows: figure 1 shown.
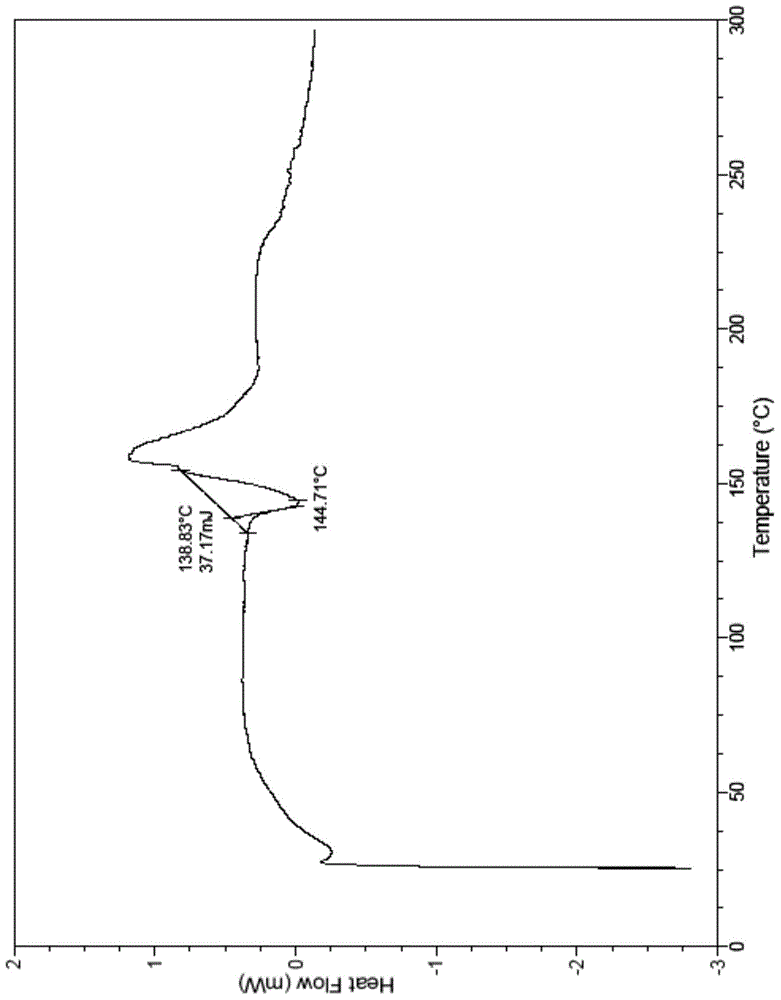
[0031] The above crystal form A2 of ibrutinib also has the follow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com