Preparation and Application of Drug Sustained and Controlled Release System with Liquid Silicone Rubber as Carrier
A liquid silicone rubber, silicone rubber technology, used in drug combination, drug delivery, endocrine system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
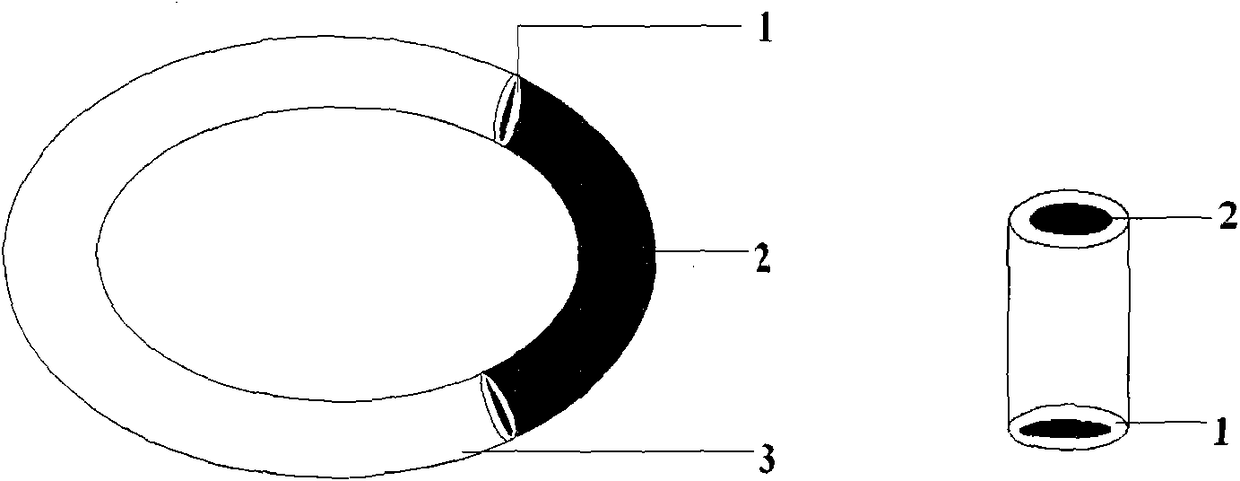
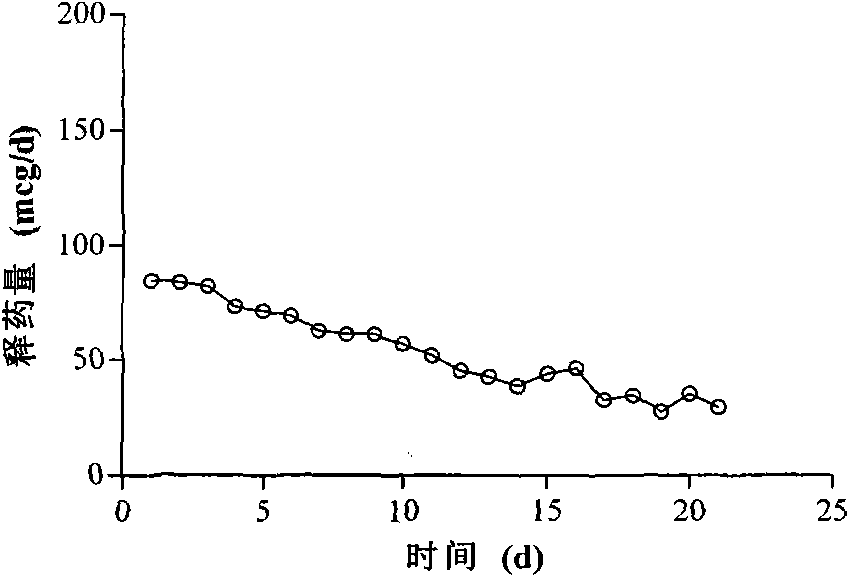
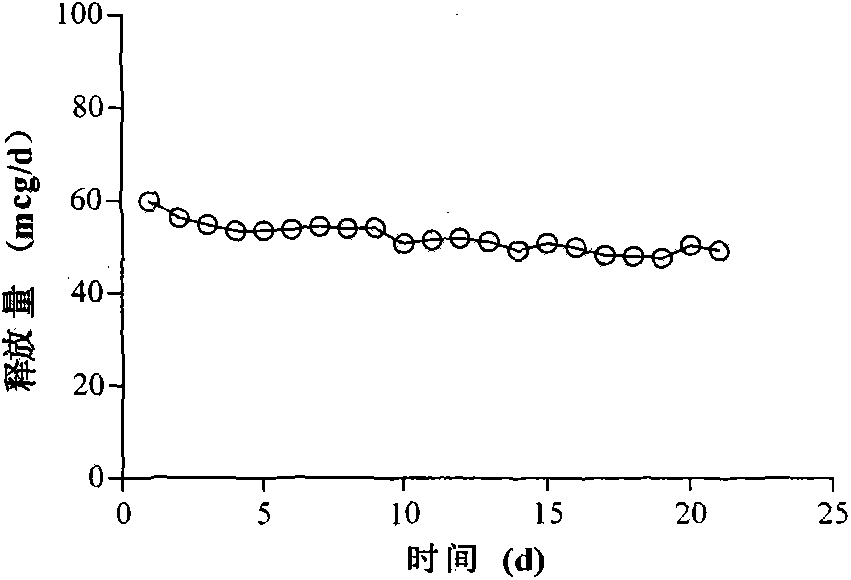
[0025] Take 100g of liquid silicone rubber MED-6382 matrix, 0.5g of cross-linking agent, stir for 30s to mix, weigh 2g of Gestodene, add it to the silicone rubber mixture and stir for 30s to fully mix the drug and silicone rubber mixture, use 50ml Transfer the appropriate amount of the mixture to the ring mold with the syringe, avoid air bubbles as much as possible during the operation, then place the mold at 80°C for 20 minutes, and remove the formed ring silicone rubber product from the mold. The outer diameter of the silicone rubber product is is 50mm, and the cross-sectional diameter is 4mm. Mix the two components of C6-165 medical silicone rubber at a ratio of 1:1 for 10 minutes to prepare a film with a thickness of 0.75mm, and wrap the film on a ring composed of 1 / 4 drug-containing skeleton and 1 / 4 blank skeleton On the surface, vulcanize at 130°C for 10 minutes to obtain a block-type reservoir-type silicone rubber rod-shaped drug release carrier, and use the slurry meth...
Embodiment 2
[0027] Take 100g of liquid silicone rubber MED-6382 matrix, 0.5g of cross-linking agent, stir for 30s to make it evenly mixed, weigh 15g of levonorgestrel and add it to the silicone rubber mixture and stir for 30s to fully mix the drug and the silicone rubber mixture, use Transfer an appropriate amount of the mixture into a rod-shaped mold with a 50ml syringe, avoid air bubbles as much as possible during the operation, then place the mold at 80°C for 20 minutes, and remove the formed rod-shaped silicone rubber product from the mold. The length of the silicone rubber product is 6.5 cm, the cross-sectional diameter is 6.5mm. Mix the two components of C6-165 medical silicone rubber according to the ratio of 1:1 for 10 minutes to prepare a film with a thickness of 0.75mm, wrap the film on the surface of the molded silicone rubber product, and vulcanize at 130°C for 10 minutes to obtain the storage type silicone rubber For the rod-shaped drug release carrier, the sample is subjecte...
Embodiment 3
[0029] Take 100g of liquid silicone rubber MED-6382 matrix, 0.5g of cross-linking agent, stir for 30s to mix, weigh 15g of acyclovir, add it to the silicone rubber mixture and stir for 30s to fully mix the drug and silicone rubber mixture, use 50ml Transfer the appropriate amount of the mixture to the rod-shaped mold with the syringe, avoid air bubbles as much as possible during the operation, then place the mold at 80°C for 20 minutes, and remove the formed rod-shaped silicone rubber product from the mold. The length of the silicone rubber product is 6.5cm , the cross-sectional diameter is 6.5mm. Mix the two components of Q7-4765 medical silicone rubber according to the ratio of 1:1 for 10 minutes to prepare a film with a thickness of 0.75mm, wrap the film on the surface of the molded silicone rubber product, and vulcanize at 130°C for 10 minutes to obtain the storage type silicone rubber For the rod-shaped drug release carrier, the sample is subjected to an in vitro release ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com