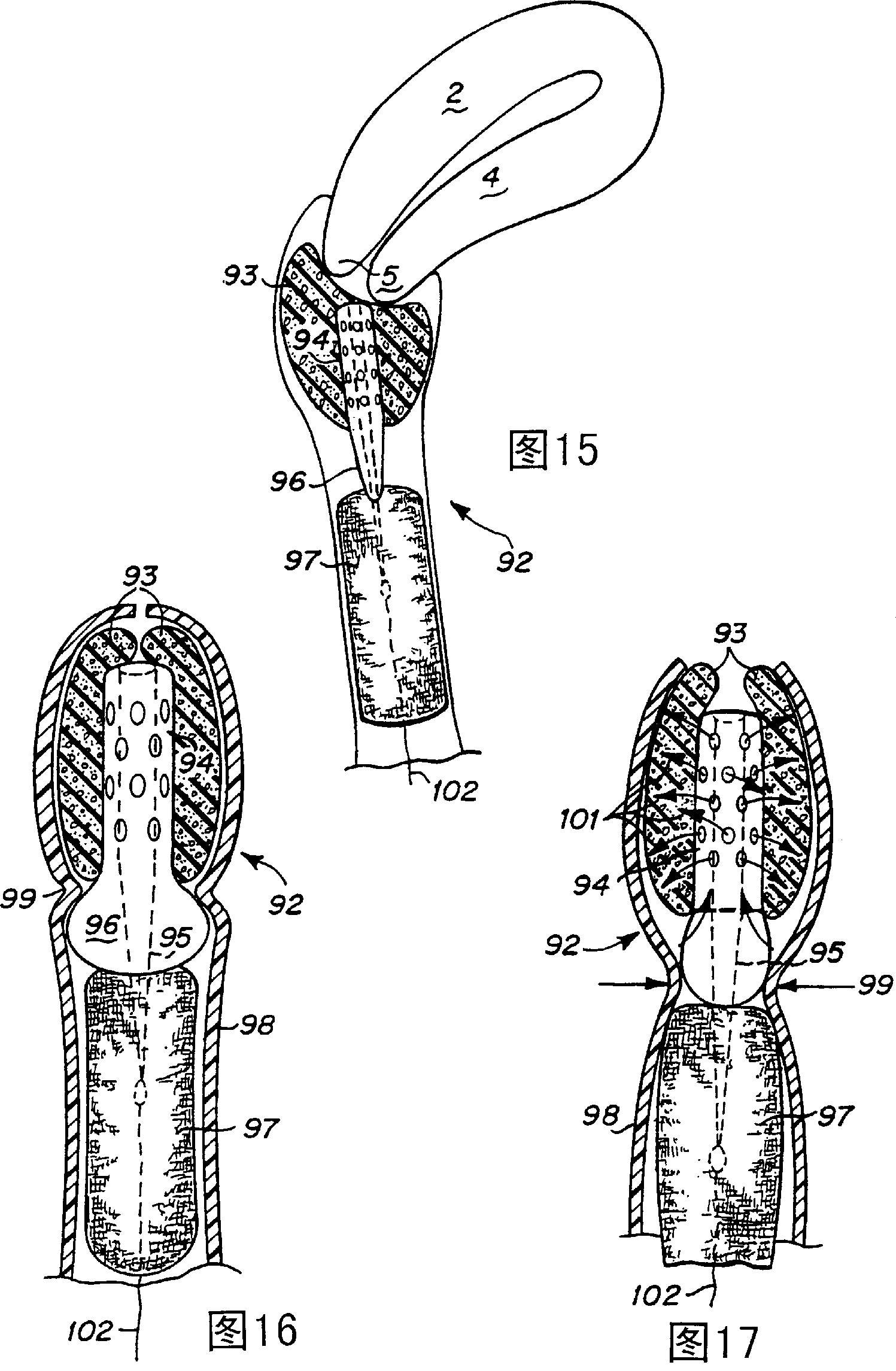
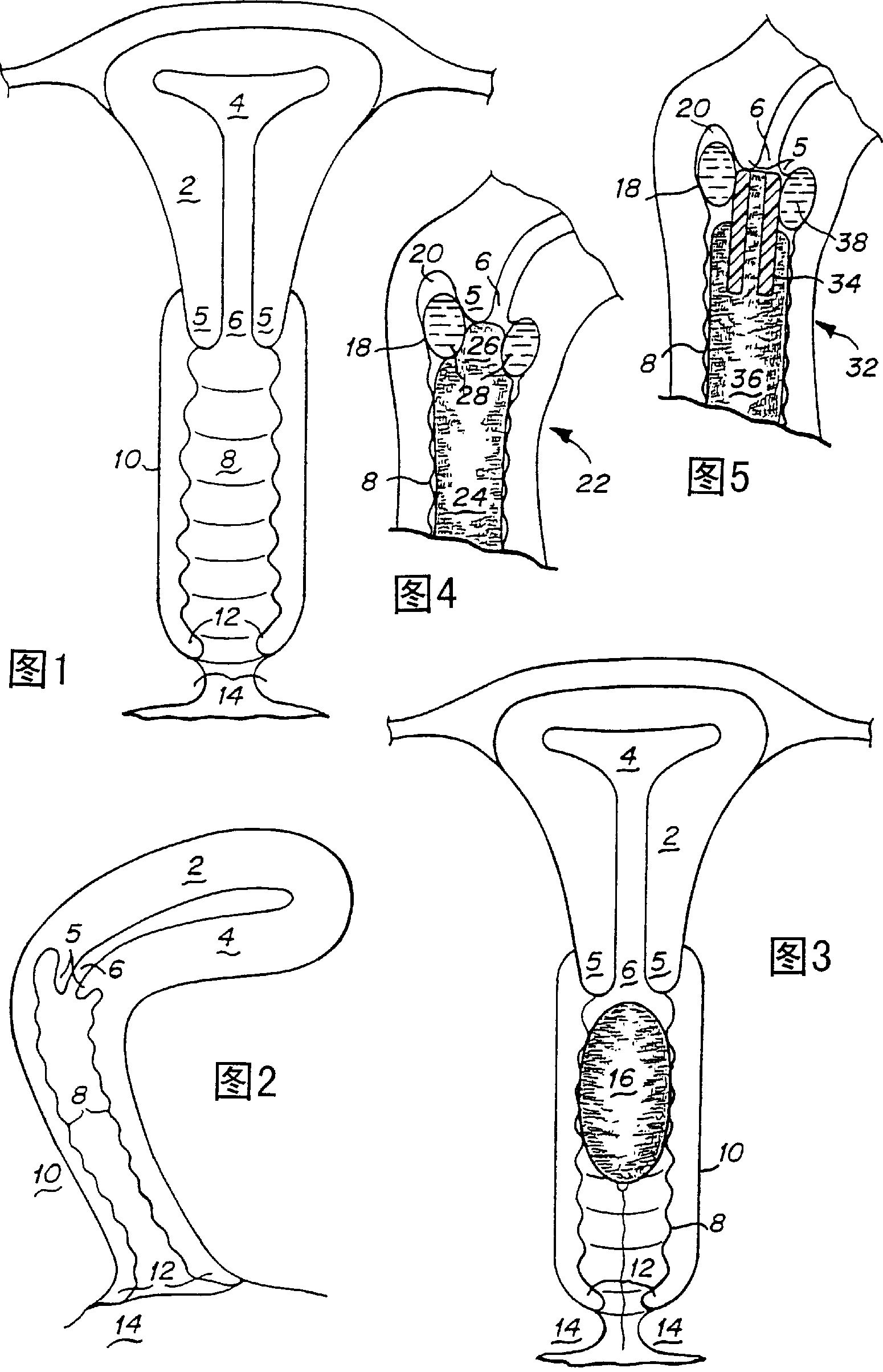
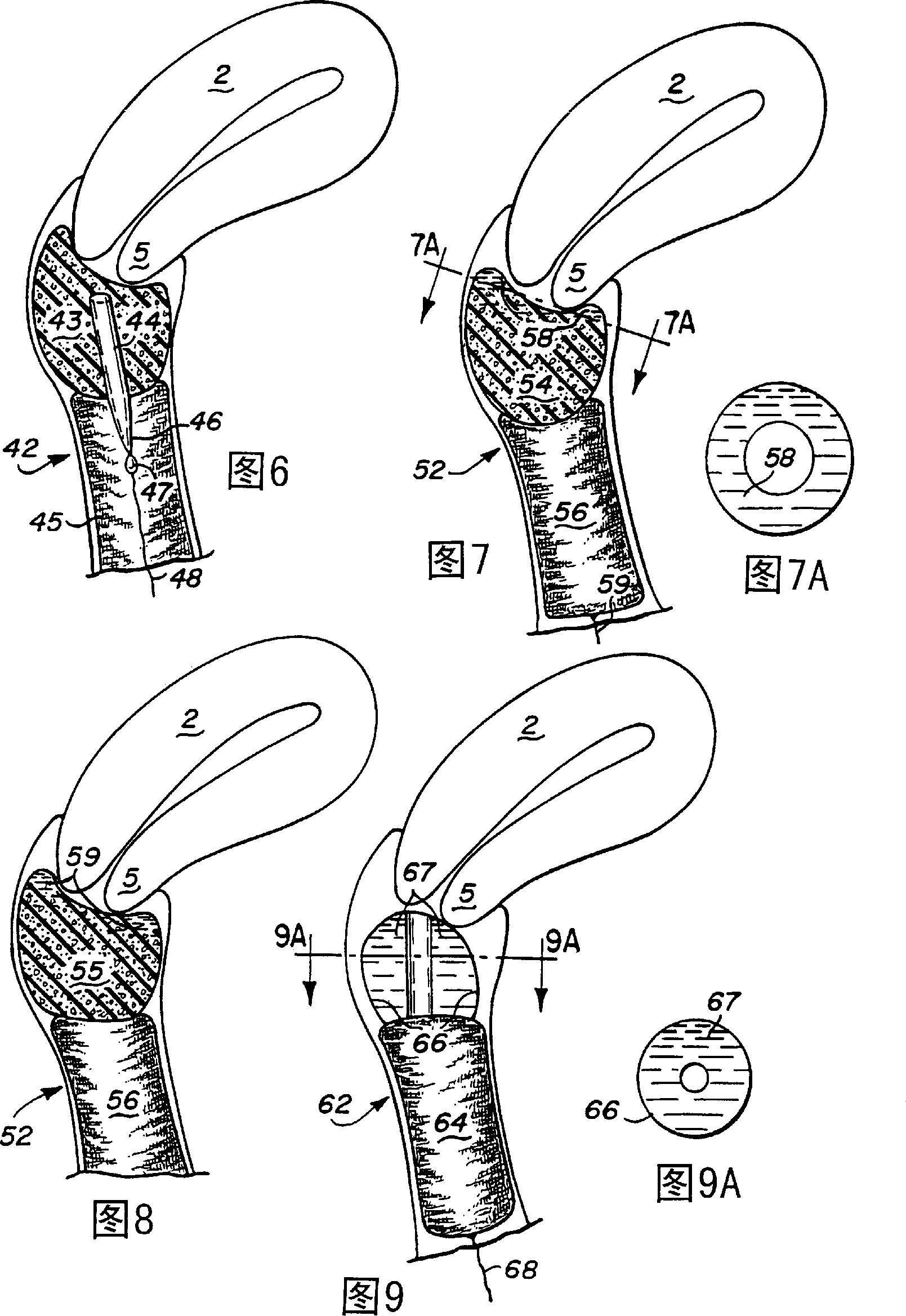
Device and method for treatment of dysmenorrhea
A medicament and a selected technology are applied in the field of devices for treating dysmenorrhea, and can solve the problems of obtaining analgesics, restricting administration, failing to successfully relieve dysmenorrhea symptoms, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Preparation of Verapamil Vaginal Suppository
[0099] The dose of verapamil (Sigma / Aldrich, St. Louis, MO) was 0.15-0.6 mg / kg body weight. Radiolabeled verapamil (4-7 μCi) was added to the unlabeled compound. Pessaries were formulated and prepared 24 hours prior to each test. The three basic components used in suppositories are: SUPPOCIRE® AS2 (Gattefosse, Westwood, NJ) (75% by weight); hydroxypropyl methylcellulose (e.g. METHOCEL® K, HPMC K15M) (Michigan Dow Chemical Company, Midland) (10% by weight), a mucoadhesive; and TRANSCUTOL(R) (Gattefosse) (15% by weight). To make 8 suppositories, weigh 4.5g SUPPOCIRE, 600mg HPMC, 900mg TRANSCUTOL, calculate the dose of the drug, and its labeled counterpart. The SUPPOCIRE was melted in a single-use 100ml polypropylene beaker suspended in water at 50°C. The solution was stirred until completely molten. Then HPMC and TRANSCUTL were added and mixed. Finally, unlabeled drug and radiolabeled drug are added to ...
Embodiment 2
[0101] Preparation of indomethacin vaginal suppository
[0102] 14 C-Indomethacin was obtained from Amersham Life Science, Arlington Hts., IL. The dose of frozen indomethacin (Sigma / Aldrich) was 0.2 mg / kg body weight. Labeled indomethacin (4-7 μCi) was added to the cooled compound. All other steps in the preparation of indomethacin suppositories are the same as in Example 1, but indomethacin is used instead of verapamil.
Embodiment 3
[0104] Pharmacokinetic test of verapamil
[0105] 3 H-Verapamil was obtained from Dupont / NEN (Boston, MA). Cold verapamil (Sigma / Aldrich, St. Louis, Missouri) (0.15–0.6 mg / kg body weight, i.v.) was dissolved in 0.5 ml of dimethylsulfoxide (Syntex, West Des Moines, IA) prior to intravenous injection . Labeled verapamil (4-7 μCi) was then added to the cold compound prior to iv injection.
[0106] Female New Zealand White rabbits weighing 2.8-3.5 kg were obtained from Myrtle Rabbitry (Thompson Station, TN). Rabbits were housed in a National Institute of Health (NIH) approved facility and acclimatized for at least 48 hours prior to each test.
[0107] Pharmacokinetic studies of the drug were performed using two models of intravenous and intravaginal administration. In the first series of experiments, the intravenous route was used to determine the initial half-life of the test compound. In a second series of experiments, intravenous and intravaginal administra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com