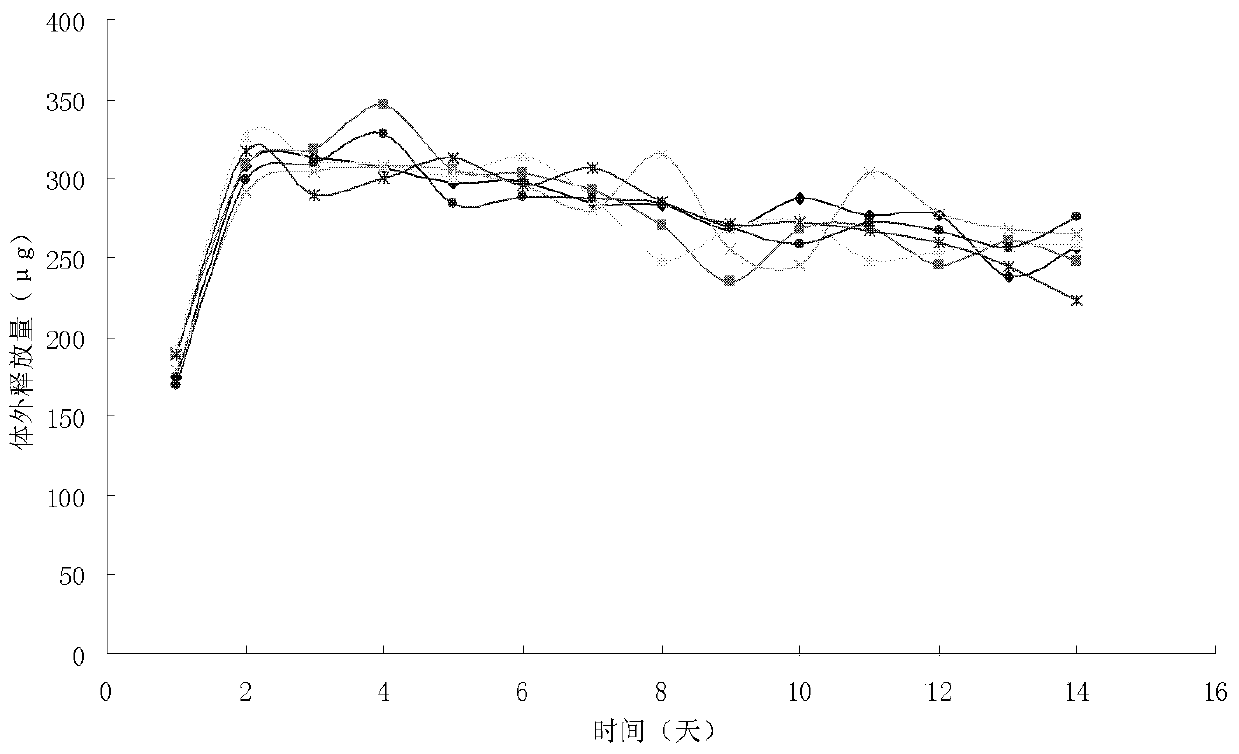
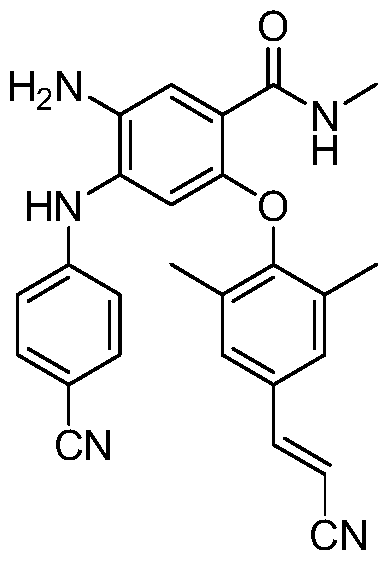
Vaginal ring containing non-nucleoside reverse transcriptase inhibitor and its preparation method
A technology of vaginal ring and beta-cyclodextrin, applied in the field of vaginal ring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Grinding and mixing 30 mg of "108-13" and 150 mg of hydroxypropyl beta-cyclodextrin with a Retsch-Rm200 mortar mill imported from Germany. Grinding in a mortar grinder for 20 minutes (grinding gap ≤ 0.3 mm), the drug molecule is completely embedded in the molecule of hydroxypropyl beta cyclodextrin to form a new inclusion body.
[0028] (2) Add 350 mg of sodium lauryl sulfate to the inclusion complex formed by the above-mentioned "108-13 and hydroxypropyl beta-cyclodextrin, continue to adopt the Retsch-Rm200 mortar grinder imported from Germany, in the mortar grinder Continue grinding for 20 minutes (grinding gap ≤ 0.3mm), combine the drug molecules and hydroxypropyl beta cyclodextrin in vitro and include a new layer of solubilizing substances.
[0029] (3) The molecular inclusion body formed by combining the above-mentioned drug, hydroxypropyl beta cyclodextrin, and sodium lauryl sulfate, and 2.2g of silicone rubber are mixed together to form a columnar body in the...
Embodiment 2
[0031] (1) Grind 40 mg of "108-13" and 80 mg of hydroxypropyl beta-cyclodextrin in a mortar grinder imported from Germany for 20 minutes (grinding gap ≤ 0.3 mm), A new inclusion complex is formed by fully embedding the drug molecule in the molecule of hydroxypropyl beta cyclodextrin.
[0032] (2) Add 200 mg of sodium lauryl sulfate to the inclusion complex formed by the above-mentioned "108-13 and hydroxypropyl beta-cyclodextrin, continue to adopt the Retsch-Rm200 mortar grinder imported from Germany, in the mortar grinder Continue grinding for 20 minutes (grinding gap ≤ 0.3mm), combine the drug molecules and hydroxypropyl beta cyclodextrin in vitro and include a new layer of solubilizing substances.
[0033] (3) The molecular inclusion body formed by combining the above-mentioned drug, hydroxypropyl beta cyclodextrin, and sodium lauryl sulfate, and 2.4g of silicone rubber are mixed together to form a columnar body in the extruder , embedded in the mold for hot press vulcaniz...
Embodiment 3
[0035] (1) Grinding and mixing 35 mg of "108-13" and 200 mg of hydroxypropyl beta-cyclodextrin with a Retsch-Rm200 mortar mill imported from Germany. Grinding in a mortar grinder for 20 minutes (grinding gap ≤ 0.3 mm), the drug molecule is completely embedded in the molecule of hydroxypropyl beta cyclodextrin to form a new inclusion body.
[0036] (2) Add 250 mg sodium lauryl sulfate to the inclusion complex formed by the above-mentioned "108-13 and hydroxypropyl beta cyclodextrin, continue to adopt the Retsch-Rm200 mortar grinder imported from Germany, Continue grinding for 20 minutes (grinding gap ≤ 0.3mm), combine the drug molecules and hydroxypropyl beta cyclodextrin in vitro and include a new layer of solubilizing substances.
[0037] (3) The molecular inclusion body formed by combining the above-mentioned drug, hydroxypropyl beta cyclodextrin, and sodium lauryl sulfate, and 2.3g of silicone rubber are mixed together to form a columnar body in the extruder , embedded in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com