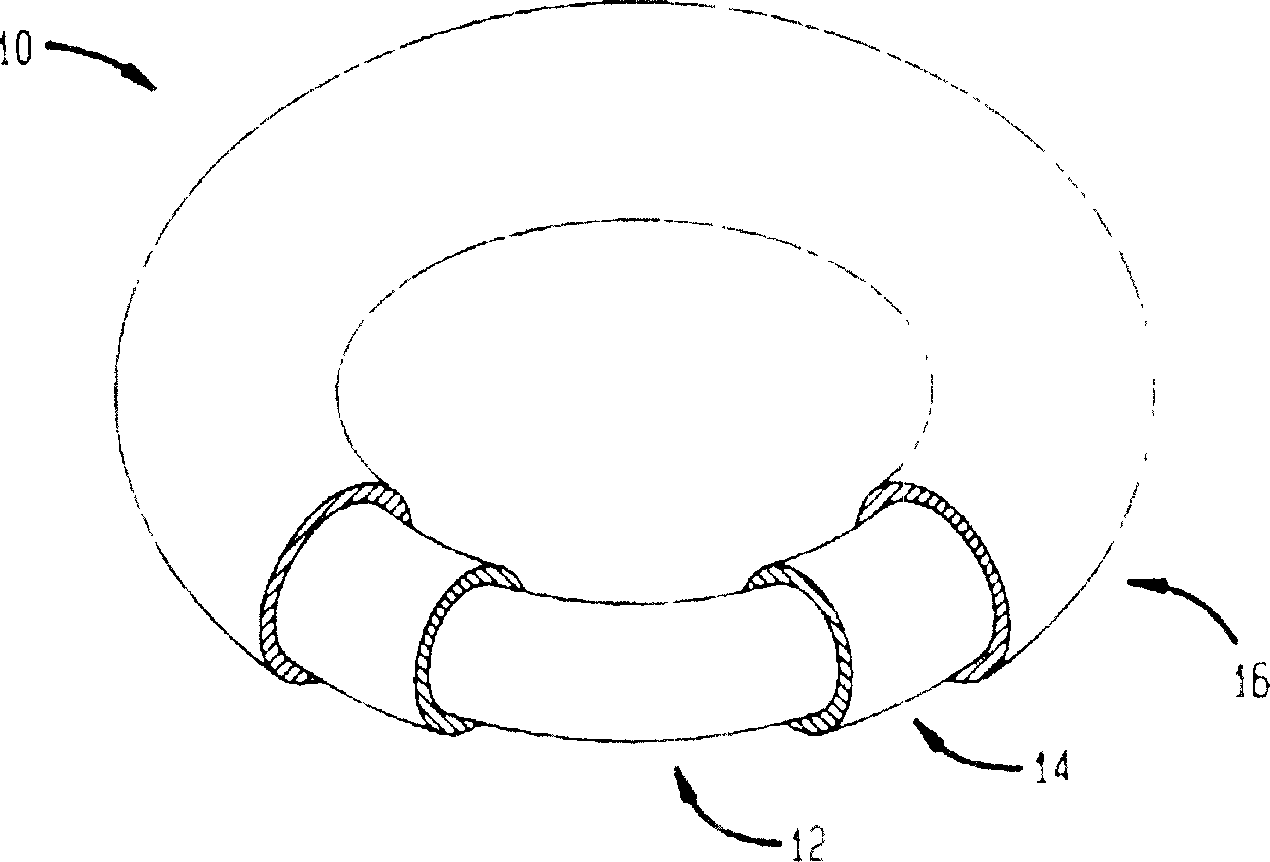
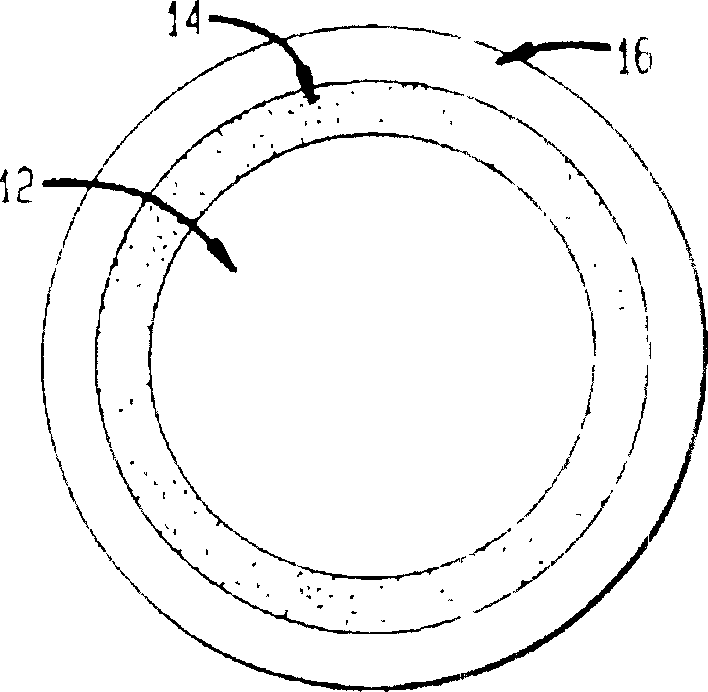
Intravaginal rings with insertable drug-containing core
A vaginal ring and drug core technology, applied in the field of intravaginal drug delivery device and vaginal drug delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Examples of burst effects and storage condition effects
[0054] Prepared with room temperature vulcanizable polydimethylsiloxane image 3 A vaginal ring that is medium but contains only one core. Stannous octoate (2-ethylhexanoate) was used as a catalyst to cause sulfidation. The annulus has dimensions of 56 mm overall diameter and 9 mm cross-sectional diameter. The core was made from the same polymer mixed with ethinyl estradiol, the steroid concentration in the core was 7%. The core size is 2.2 x 20mm.
[0055] The vaginal rings were prepared in a two-step process, with the core in the center of the first half-ring, and the rings were then coated with polydimethylsiloxane to complete the rings. As shown in Table 1, the release rate was determined in vitro after storage to make Image 6 . At each time with temperature, 4 rings were measured.
[0056] Table 1
[0057] In vitro release rate of ethinyl estradiol during stora...
Embodiment 2
[0072] Example of using the intravaginal drug delivery device of the present invention to avoid sudden release of steroids
[0073] A vaginal ring of the present invention was prepared with 2 grooves. The ring has an overall diameter of 56mm and a cross-sectional diameter of 8.4mm. The elastomer used in the molded ring was a platinum catalyzed dimethylsiloxane / methylvinylsiloxane copolymer manufactured by Applied Silicone Corp. of Ventura, CA. This elastomer, called LSR 25-10:1, was packaged in two parts and mixed together in a 10:1 ratio to cause the vulcanization reaction.
[0074] The mixture is poured into a mold with movable steel rods to create slots for inserting the core. Vulcanization was carried out at 105°C for 30 minutes. The core was made by mixing the steroid with room temperature curable polydimethylsiloxane (MED6382 from Nusil Silicone Technology of Carpinteria, CA.). Two cores are prepared and inserted into each ring. Each is 3×15mm. A 50% NESTORONE TM ...
Embodiment 3
[0076] Example of Avoiding Sudden Release of Steroids Using the Invention
[0077] The vaginal rings of the present invention are prepared using a platinum catalyzed dimethylsiloxane / methylvinylsiloxane copolymer called LSR 25-10:1 manufactured by Applied Silicone Corp. of Ventura, CA. The ring has an overall diameter of 56mm and a cross-sectional diameter of 8.4mm. The two parts of the elastomeric composition were mixed together in a ratio of 10:1 and poured into a mold with movable steel rods to create grooves for inserting the cores. Heating at 105°C for 30 minutes for vulcanization. Combining ethinyl estradiol and NESTORONE TM The core was made by mixing with stannous octoate catalyzed polydimethylsiloxane (R-2602 manufactured by Nusil Silicone Technology of Carpinteria, CA). The core contains 12% ethinyl estradiol and 40% NESTORONE TM . The core has a diameter of 3mm and a length of 15mm or 20mm. The groove in the ring was partially filled with polydimethylsiloxane ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Overall diameter | aaaaa | aaaaa |
| Cross section diameter | aaaaa | aaaaa |
| Cross section diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com