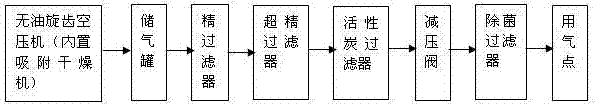
Sterile injection water production technique and sterile compressed air preparation method
A water for injection and production process technology, applied in chemical instruments and methods, medical preparations of non-active ingredients, pharmaceutical formulas, etc., can solve problems such as potential safety hazards, achieve safety assurance, improve sterility assurance levels, and control heat sources The effect of content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
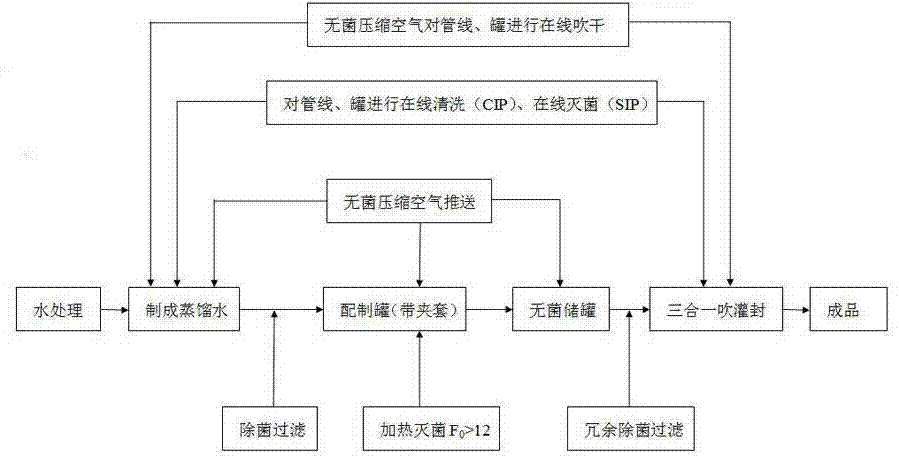
[0029] Such as figure 1 As shown, the production process of sterile water for injection of the present invention is firstly to carry out online cleaning CIP and online sterilization SIP to all pipelines, preparation tanks and storage tanks used for the production of sterile water for injection with distilled water or pure steam.
[0030] The online cleaning CIP is to keep the pressure under the condition of 0.15MP sterile compressed air for 15 minutes, and then perform pre-washing; after pre-washing, use 300kg of distilled water with a temperature above 80°C to rinse and sterilize the storage tank for more than 15 minutes; then use 80°C distilled water, online rinse at a pressure of 0.25 MP for more than 15 minutes, and finally use sterile compressed air to dry at a pressure of 0.15 MP for 15 minutes.
[0031] The online sterilization SIP requires pure saturated steam detection first, that is, to detect the steam generated by the pure steam generator. When the steam reaches 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com