Preparation method of arginine aspirin and powder-injection of arginine aspirin
A technology of arginine aspirin and aspirin, applied in the direction of medical preparations containing active ingredients, anti-inflammatory agents, powder delivery, etc., can solve the problems of cumbersome steps, prolonged time, low crystallization temperature, etc., and achieve easy industrial production and reaction The effect of short process and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
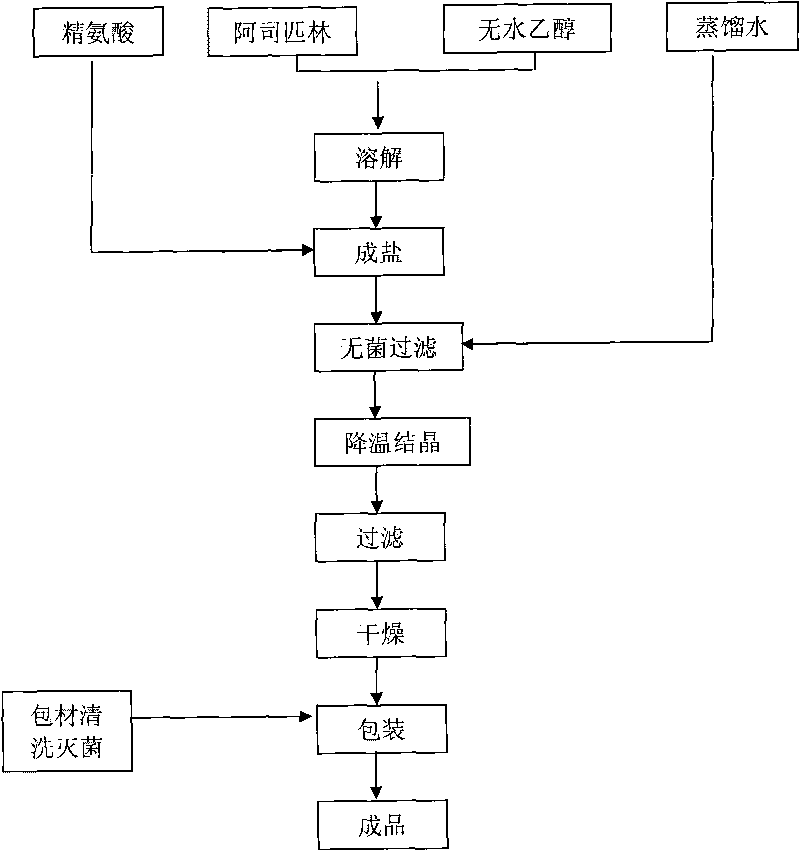
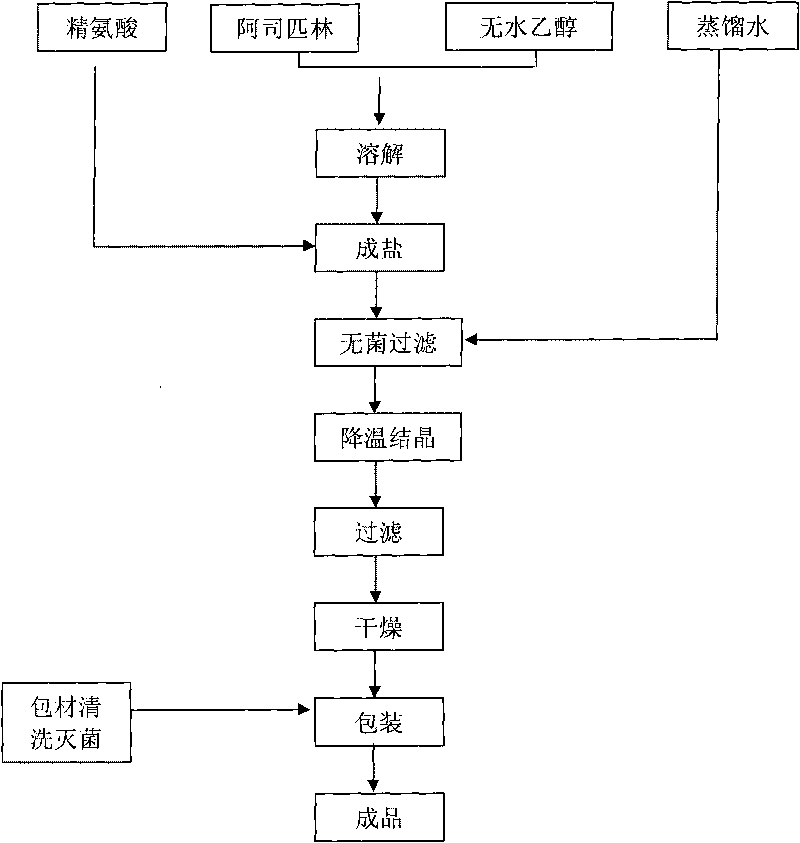
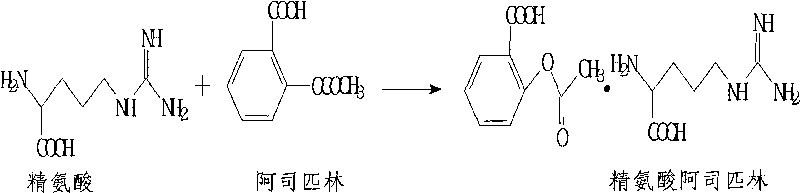
[0027] Dissolve 40.0g of aspirin in 120ml of absolute ethanol, add 35.2g of arginine to react for 20 minutes at 40°C under agitation, then add 20ml of distilled water, and when the solution is clear, filter it aseptically and cool down to 29°C Insulated and stirred for 5 hours, then slowly cooled to 10°C for suction filtration, then fully washed twice with absolute ethanol, and vacuum-dried at 35°C for 6 hours to obtain 57.5 g of sterile arginine aspirin, with a total yield of about 75.2%. And the product quality fully complies with the national standard. Then the aspirin arginine aspirin powder for injection can be obtained by aseptic subpackaging.
[0028] The obtained arginine aspirin powder injection is detected, and the results are shown in Table 1:
[0029] The detection result of table 1 embodiment 1
[0030] Loss on drying
Embodiment 2
[0032] Dissolve 80g of aspirin in 400ml of absolute ethanol, add 77.3g of arginine at 30°C to react for 30 minutes under stirring conditions, then add 44ml of distilled water, after the solution is clarified, filter it aseptically, cool down to 25°C and keep stirring 6 hours, then slowly cool down to 8°C for suction filtration, then fully wash twice with absolute ethanol, and vacuum-dry at 35°C for 8 hours to obtain 122.2g of sterile arginine aspirin, with a total yield of about 77.7%, and the product The quality is in full compliance with national standards. Then the aspirin arginine aspirin powder for injection is obtained through aseptic subpackaging.
[0033] The obtained arginine aspirin powder injection is detected, and the results are shown in Table 2:
[0034] The detection result of table 2 embodiment 2
[0035] Loss on drying
Embodiment 3
[0037] Dissolve 60g of aspirin in 480ml of absolute ethanol, add 55.2g of arginine at 30°C to react for 40 minutes while stirring 8 hours, then slowly cool down to 5°C and filter with suction, then fully wash the crystals 3 times with absolute ethanol, and dry in vacuum at 30°C for 8 hours to obtain 93.3g of sterile arginine aspirin, with a total yield of about 81.0%, and Product quality is in full compliance with national standards. Then the aspirin arginine aspirin powder for injection is obtained through aseptic subpackaging. The obtained arginine aspirin powder injection is detected, and the results are shown in Table 3:
[0038] The detection result of table 3 embodiment 3
[0039] Loss on drying
[0040] Obviously, the content of free salicylic acid in the powder injection prepared by the method of the present invention is very low, the content of active ingredients is high, the weight loss on drying is low, and the stability is strong.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com