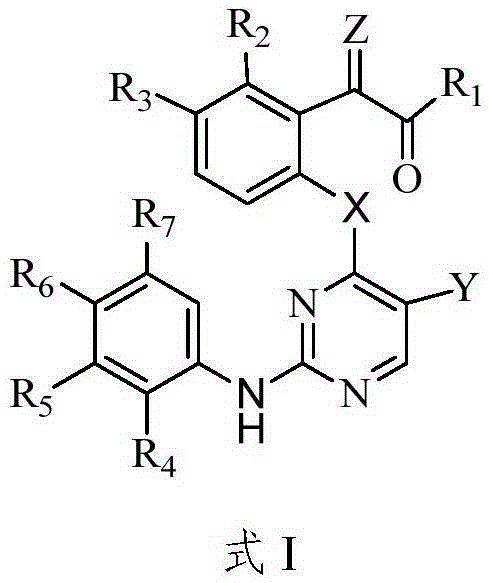
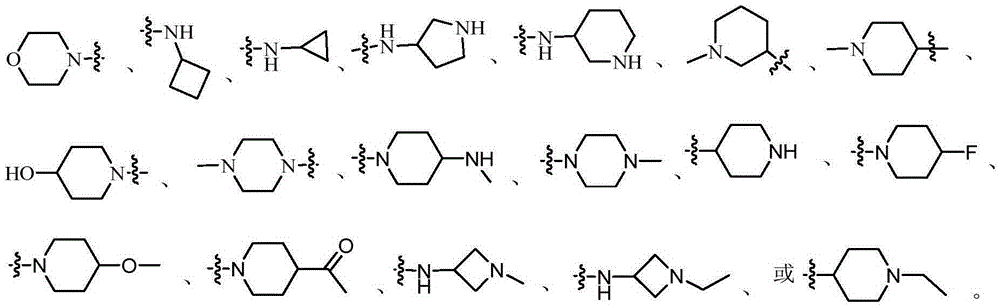
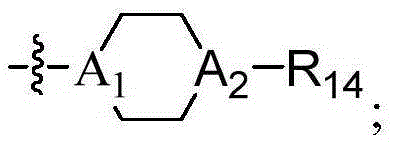Protein tyrosine kinase modulators and methods of use
A technology of selection and pharmacy, applied in the field of pyrimidine compounds, it can solve the problems such as the inability to increase the plasma level of the dose and the small effect of wild-type EGFR
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0333] The synthesis of embodiment 1 compound 1
[0334]
[0335] Compound 1a (25g, 0.11mol), Compound 1b (20g, 0.11mol) and K 2 CO 3 (37.5g, 0.22mol) was dissolved in DMF (200ml), and the mixture was stirred at 70°C for 3 hours. Then water (300ml) was added to the mixture, extracted with EA (200ml×3), HCl (1mol / L) was added to the aqueous phase to adjust the pH to 3-4, filtered, and the precipitate was collected and washed with methanol (5ml). After drying in air for 5 hours, 23 g of compound 1c were obtained.
[0336] Under nitrogen protection, put (COCl) in an ice bath 2 (7.2ml(COCl) 2 dissolved in 10 ml DCM) was added to a DCM solution containing compound 1c (5.0 g) and 2 drops of DMF, and stirred. After 3.5 hours the solvent was removed, the residue was dissolved in DCM and 10 ml of ethanol was added. After stirring for 1 hour, the reaction mixture was quenched with water. The organic phase was separated, dried and concentrated. The residue was purified by chro...
Embodiment 2
[0338] The synthesis of embodiment 2 compound 2
[0339]
[0340] A methanol solution of compound 1 and NaOH (0.5 ml, 2 mol / L) was stirred at room temperature for 1 hour. HCl was then added to adjust the solution to a pH of approximately 5. Filtration and drying of the solid afforded compound 2 (37 mg). MS:524.2(M+H) + .
Embodiment 3
[0341] The synthesis of embodiment 3 compound 3
[0342]
[0343] Compound 2 (140mg) and NH 2 A mixture of OH (72mg), PyBOP (180mg) was dissolved in 10ml DMF and stirred at 45°C for 3 hours. The reaction solution was purified by preparative HPLC to obtain 30 mg of solid, namely compound 3. MS:539.2(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com