Glucagon analog for treatment of metabolic diseases
A technology of glucagon and analogs, applied in the field of biological drugs, can solve problems such as difficulties, and achieve the effects of good enzyme resistance stability, long in vivo half-life and continuous action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Example 1: General Preparation and Purification Method of Glucagon Analogs
[0132] Using existing technology, such as existing literature ( V. et al., Beilstein J.Org. Chem., 10:1197–1212 (2014); Palomo, J.M., RSC Adv., 4:32658-32672 (2014); Behrendt, R. et al., J.Pept. Sci., 22:4-27 (2015)) in the polypeptide solid-phase synthesis and modification method to prepare each polypeptide involved in this patent.
[0133] Specifically, solid phase peptide synthesis can be performed on a CEM Liberty peptide synthesizer using the standard Fmoc method.
[0134] Before use, the Rink Amide TentaGel S Ram resin (0.25mmol / g, 1g) was swelled in NMP (10mL), added to the solid-phase synthesis device, and piperidine / DMF (20%, 10mL) was added to the resin to react for 30min Remove Fmoc protection, suck dry, wash with DMF (5×10 mL), and suck dry. Add the first Fmoc-amino acid solution (0.2M, NMP / DMF / DCM, 1:1:1, 5mL), COMU / NMP (0.5M, 2mL) and DIPEA / DMF (2.0M; 1mL), react at room tempera...
Embodiment 2
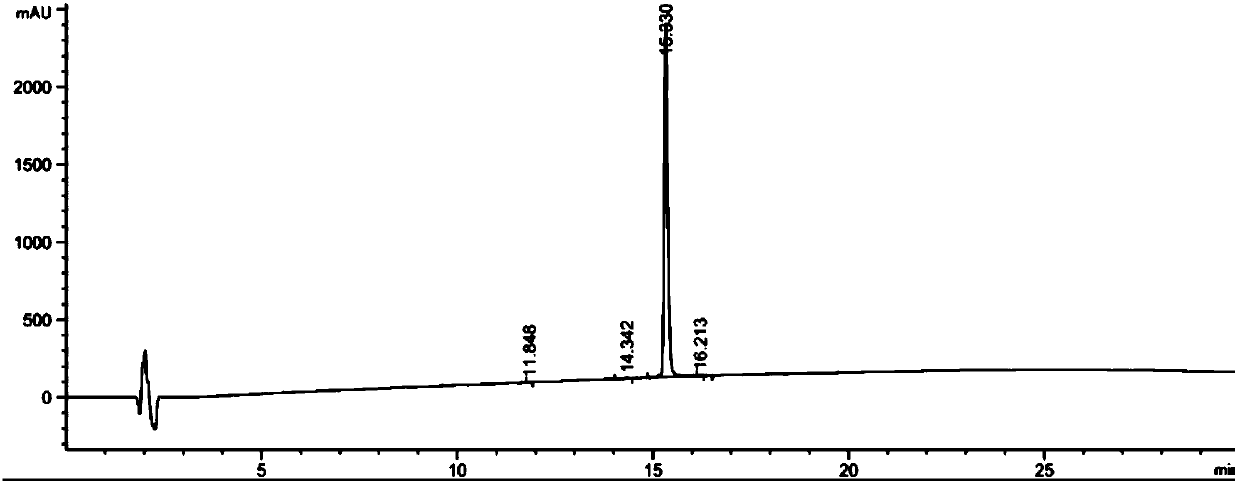
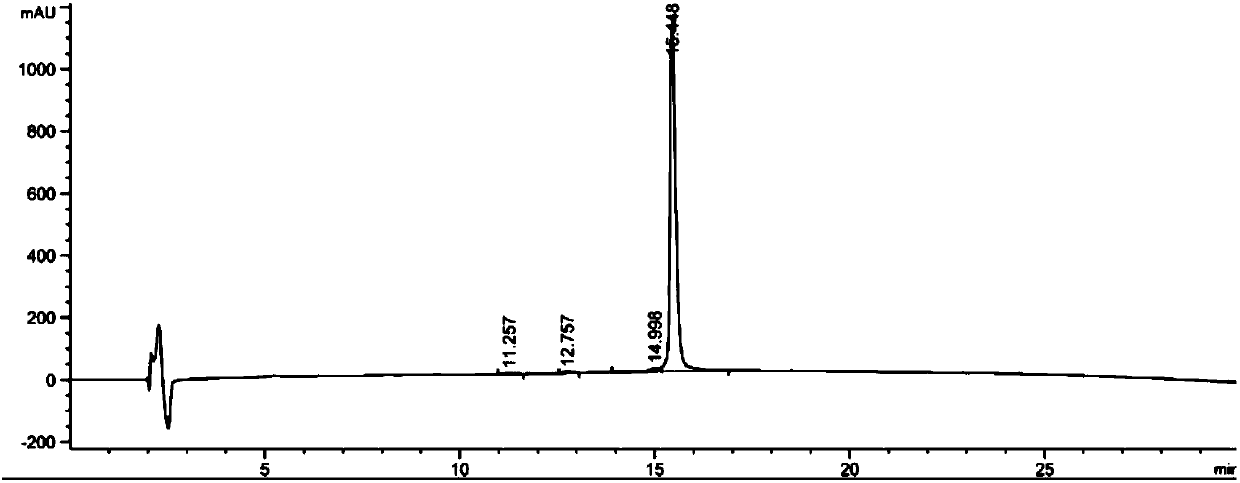
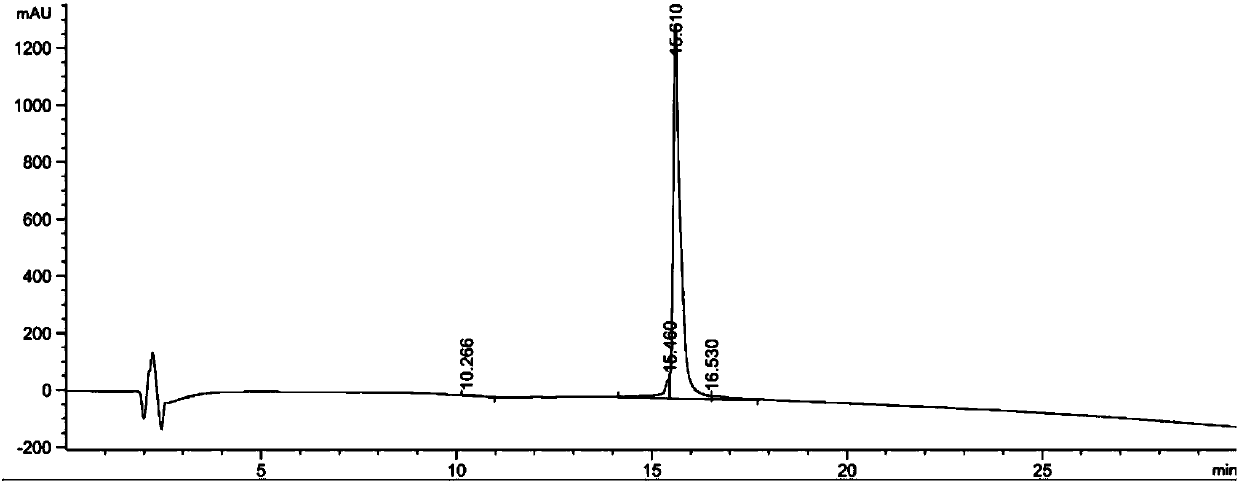
[0156] Example 2: Stability Study
[0157] The purpose of this example is to study the chemical stability of various glucagon analogues prepared in Example 1 in aqueous solution.
[0158] The polypeptide to be tested (glucagon analogue) and the reference substance were prepared in 20mM phosphate buffer PB or acetate buffer at the corresponding pH, the final concentration of the polypeptide was 0.2mg / ml and filtered through a sterile filter (0.22μm , MilliporeSLGP033RB) were filter sterilized. The prepared polypeptide solution was placed at 40°C for 7 days. Then centrifuge at 4500 rpm for 20 minutes and analyze the supernatant using RP-HPLC-UV (t7). The amount of remaining intact peptide was determined and unincubated samples (t0) were analyzed in parallel. Comparing the peak areas of the target compound at t0 and t7, the "residual peptide %" was obtained according to the following equation:
[0159] Residual peptide content %=[(peptide peak area t7)×100] / peptide peak area ...
Embodiment 3
[0184] Example 3: Serum Stability
[0185] (1) The corresponding polypeptides in Table 1 were prepared with 5mM Tris-HCl, pH8.5, 0.02% TWEEN80 solution to a concentration of 1.0mg / ml, and after sterile filtration (0.22μm, Millipore SLGP033RB), diluted with rat serum 10 times, mix well, and distribute into sterile centrifuge tubes;
[0186] (2) Take 3 tubes of each of the above samples and store them frozen at -20°C as a control, and put the rest in a 37°C incubator, and take samples at different time points to detect the activity;
[0187] (3) Using the method shown in Example 4, the agonistic activity of the polypeptide GCGR was detected.
[0188] Relative activity: take the activity value at 0 hour as 100%, and compare the values measured at subsequent time points with it. Analysis of experimental results: from Table 4 and Figure 8A and 8B Serum stability can be derived.
[0189] Table 4
[0190]
[0191]
[0192] Note: N.D. means lower than the detection limi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com