Method for preparing high-quality low molecular parnaparin sodium
A panaparin and low-molecular-weight technology, which is applied in the field of preparation of high-quality low-molecular-weight panaparin, can solve the problems of insufficient purity of low-molecular-weight heparin samples, affect the stability of preparations, and non-concentrated molecular weight distribution, and achieve half-life in vivo Long, advanced technology and feasible, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
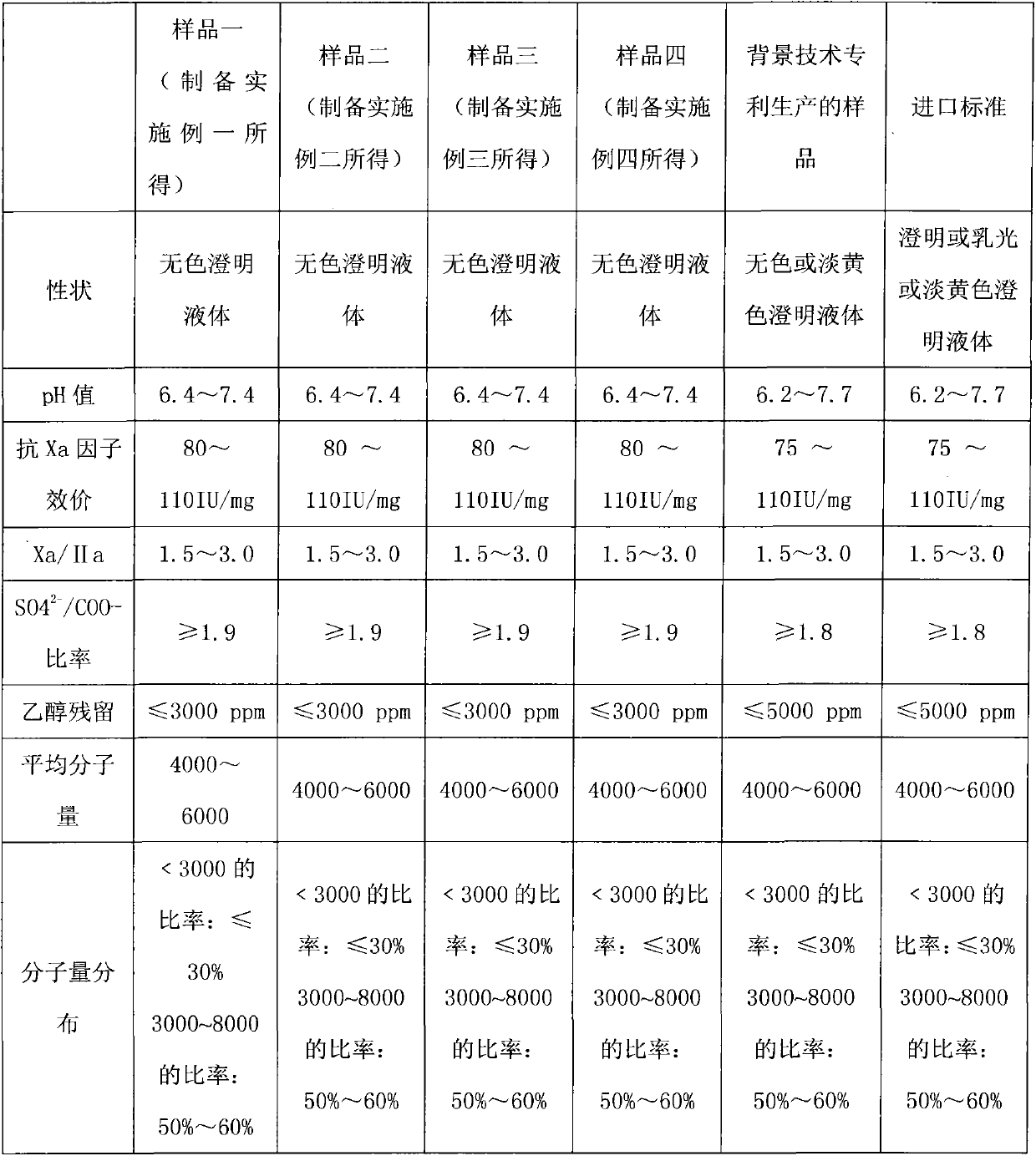
preparation Embodiment 1
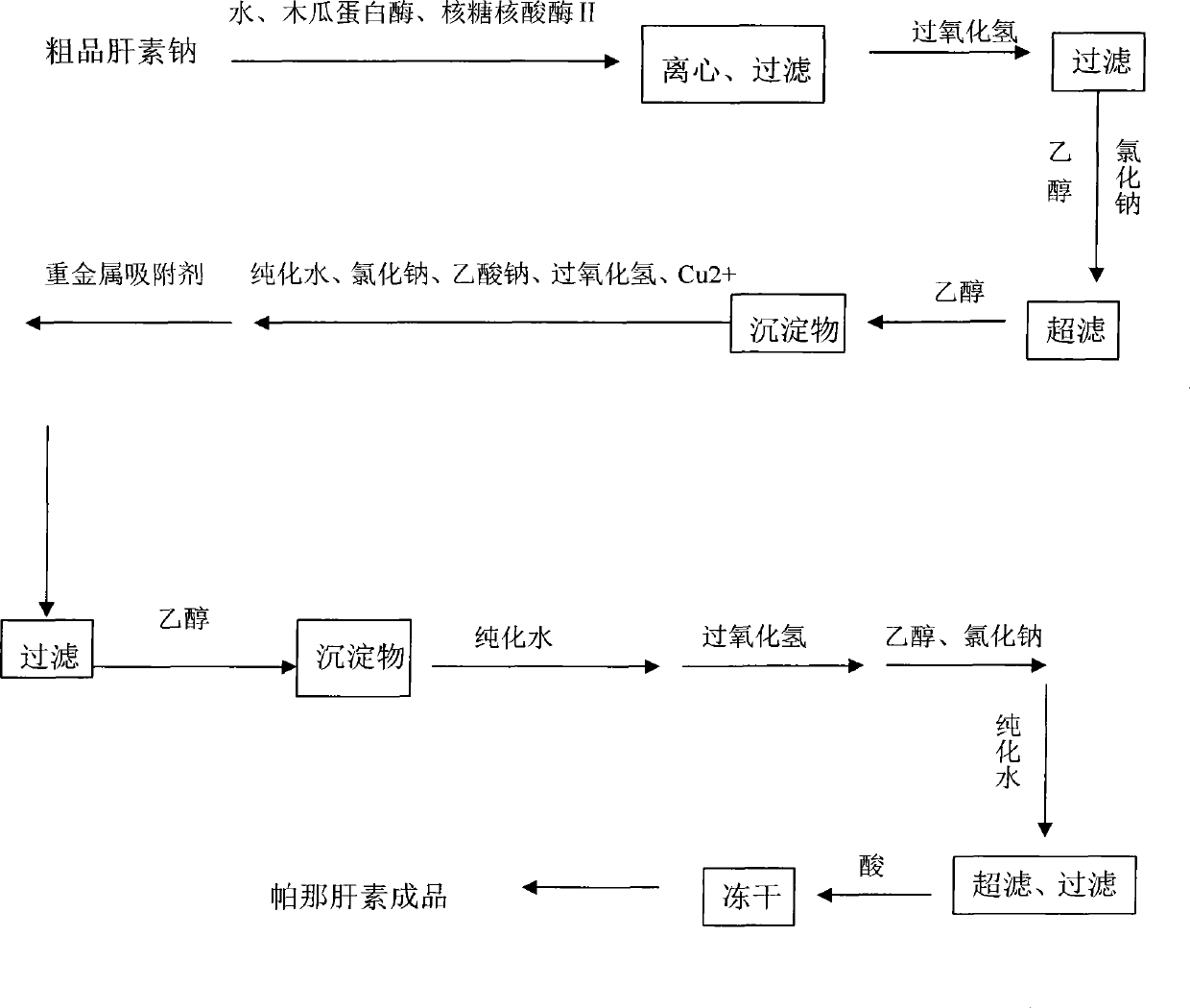
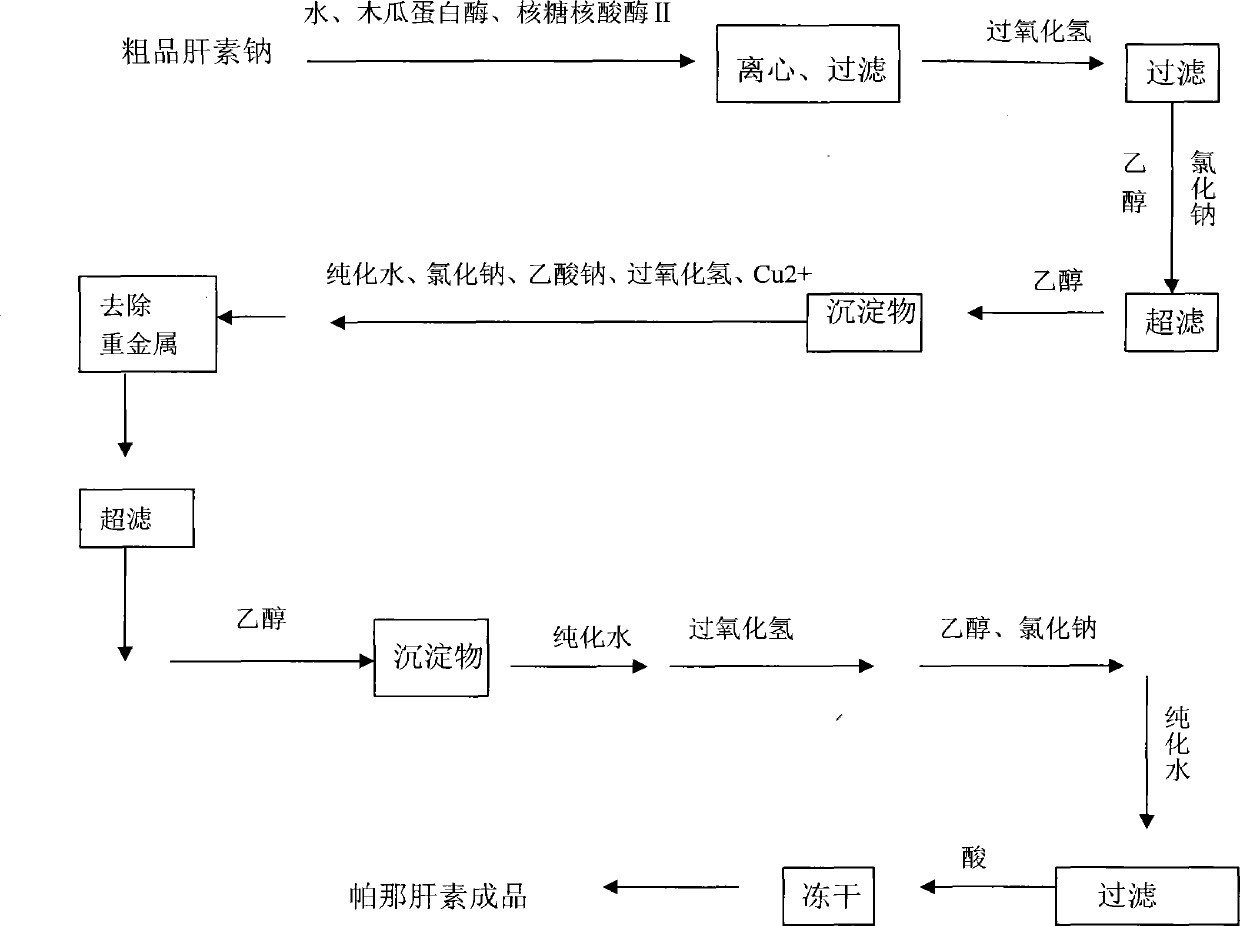
[0041] a) Enzymolysis: Take 800 g of crude heparin sodium, add 8 kg of water to dissolve it, adjust the pH of the feed solution to 8.4 with 20% (w / v) sodium hydroxide solution, and control the temperature of the feed solution to 37°C. Add 42g of papain and 36g of ribonuclease II to the feed solution, stir for 8 hours, centrifuge to remove the precipitate, filter the supernatant with a plate and frame filter, and collect 7.6L of filtrate;
[0042]b) Oxidation: control the temperature of the filtrate in step a) to 32°C, adjust the pH of the feed liquid to 9.7, add 35ml of hydrogen peroxide, stir and react for 5 hours under temperature control and pH control, and obtain a purified solution; after the oxidation is completed, use 4mol / L The HCl adjustment feed liquid pH is 6.9, after adding 72g sodium chloride wherein to dissolve, filter with the millipore filter of 0.65um, add after filtering and add 8L95% ethanol in the filtrate, leave standstill 10 hours after stirring. After st...
preparation Embodiment 2
[0051] a) Enzymolysis: Take 1000 g of crude heparin sodium, add 12 kg of water to dissolve it, adjust the pH of the feed solution to 8.2 with 20% (w / v) sodium hydroxide solution, and control the temperature of the feed solution to 36°C. Add 60 g of papain and 48 g of ribonuclease II to the feed solution, stir for 9 hours, centrifuge to remove the precipitate, filter the supernatant with a plate and frame filter, and collect 972 L of filtrate;
[0052] b) Oxidation: control the temperature of the filtrate in step a) to 36°C, adjust the pH of the feed liquid to 10.2, add 46ml of hydrogen peroxide, and stir and react for 5 hours under temperature and pH control to obtain a purified solution; after the oxidation is completed, use 4mol / L The HCl adjustment feed liquid pH is 7.1, after adding 90g sodium chloride wherein to dissolve, filter with the millipore filter of 0.65um, add after filtering and add 11L95% ethanol in the filtrate, leave standstill 10 hours after stirring. After ...
preparation Embodiment 3
[0061] a) Enzymolysis: Take 800 g of crude heparin sodium, add 8 kg of water to dissolve it, adjust the pH of the feed solution to 8.1 with 20% (w / v) sodium hydroxide solution, and control the temperature of the feed solution to 36°C. Add 45g of papain and 36g of ribonuclease II to the feed solution, stir for 8 hours, centrifuge to remove the precipitate, filter the supernatant with a plate and frame filter, and collect 7.8L of the filtrate;
[0062] b) Oxidation: Control the temperature of the filtrate in step a) to 34°C, adjust the pH of the feed liquid to be between 9.8, add 26ml of hydrogen peroxide, and stir and react for 4.5 hours under temperature and pH control to obtain a purified solution; after oxidation, use 4mol The HCl of / L regulates feed liquid pH to be 6.8, after adding 63g sodium chloride wherein to dissolve, filter with the millipore filter of 0.65um, add again after filtering, add 8L95% ethanol in the filtrate, leave standstill 10 hours after stirring . Af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com