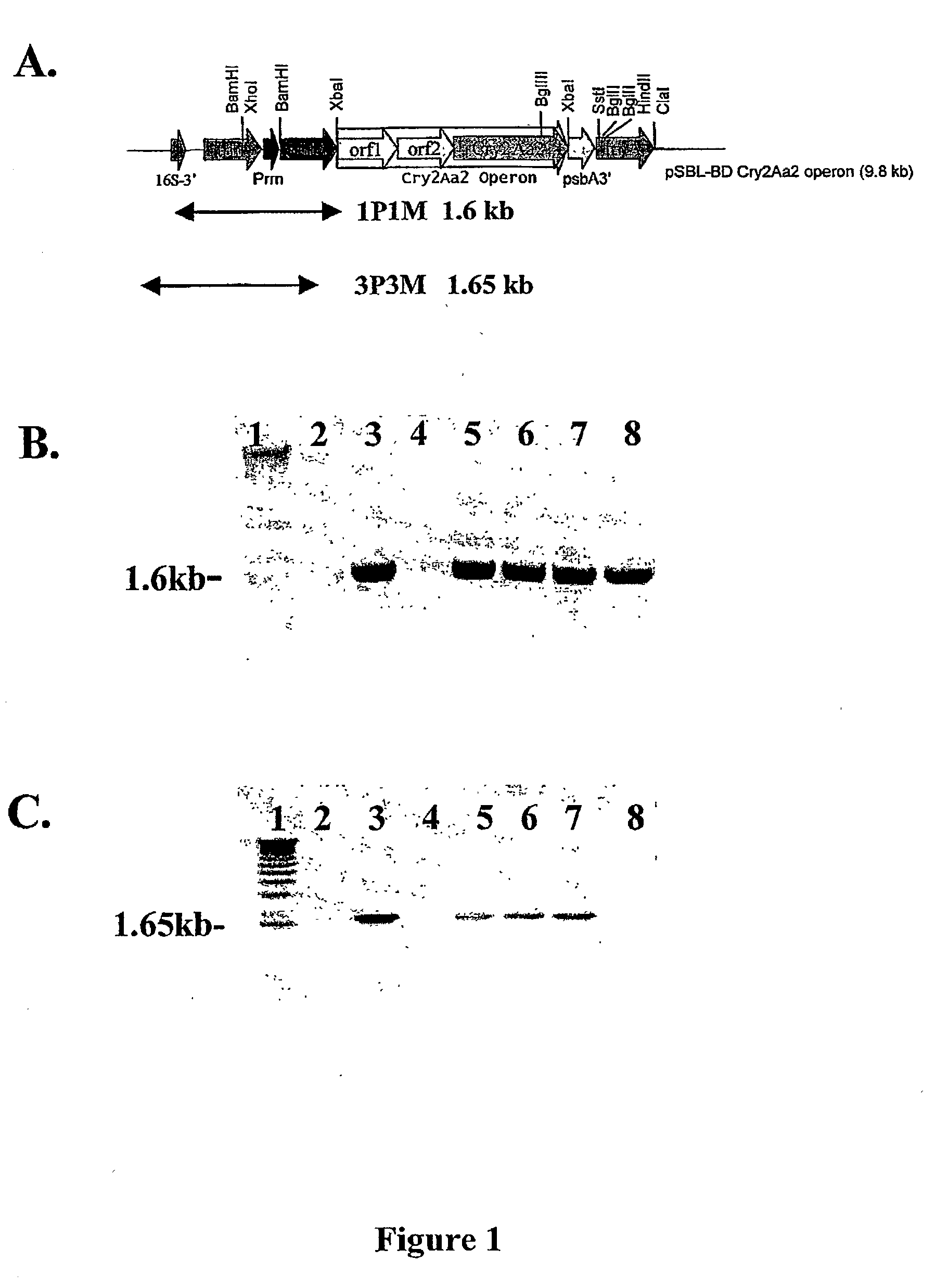
Mutiple gene expression for engineering novel pathways and hyperexpression of foreign proteins in plants
a technology of foreign protein and gene expression, applied in the field of mutation gene expression for engineering novel pathways and hyperexpression of foreign proteins in plants, can solve the problems of increasing the toxicity of organomercurials, threatening the safety, and other techniques, such as mechanical and bacterial bioremediation, which have little success in implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
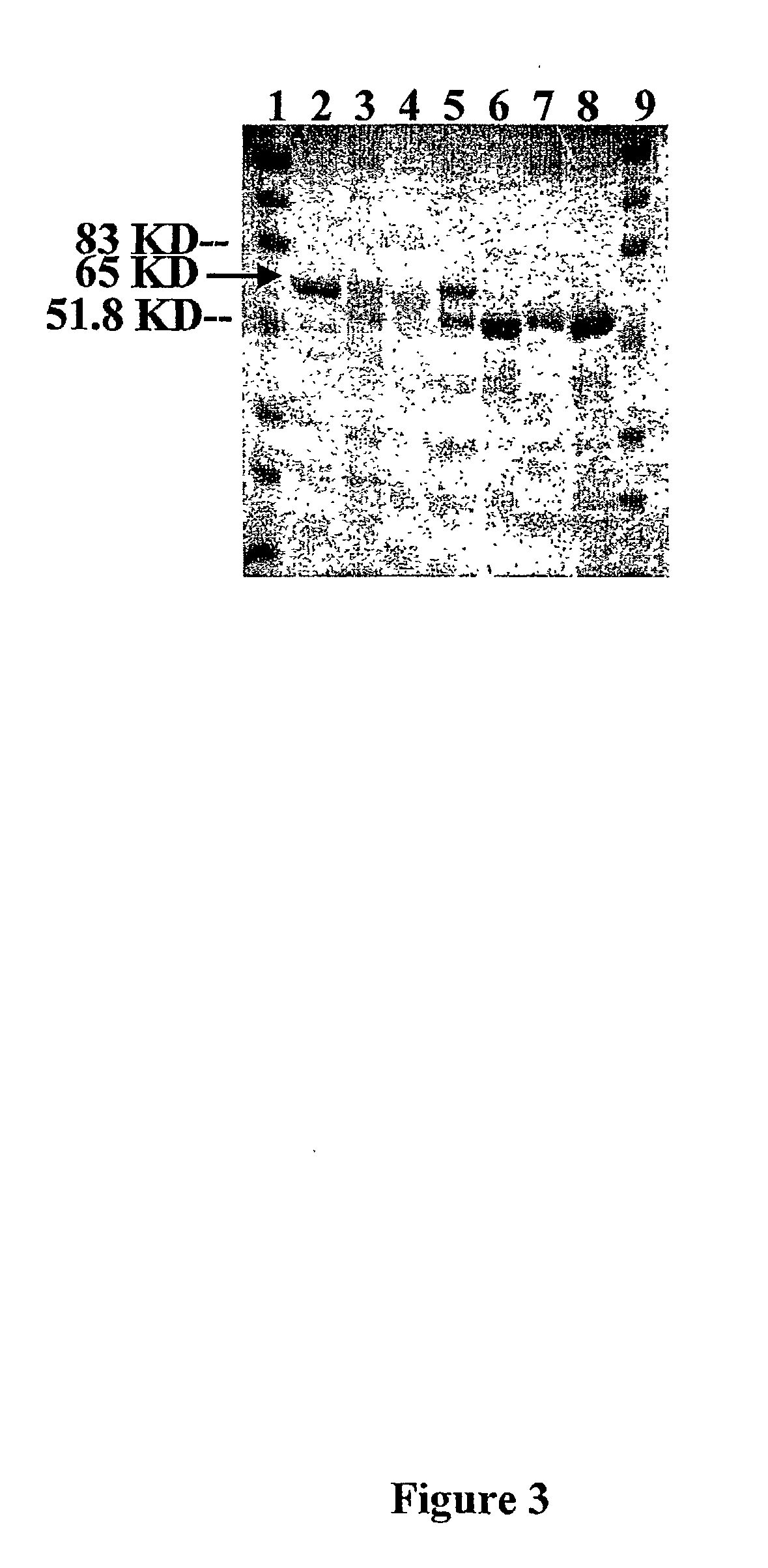
[0123] E. Coli Transformants. Due to the similarity of protein synthetic machinery (Brixey et al. 1997), expression of all metal resistance conferring chloroplast vectors are first tested in E. coli before their use in tobacco transformation The activity of the enzymes, mercury ion reductase (merA) and organomercurial lyase (merB) are tested by transforming E. coli (XLI-blue) with the recombinant plasmids and growing them in LB solid medium with HgCl.sub.2 (FIG. 9). The cells, control (XLI-bue), pLD-merAB and pLDmerAB-3'UTR are grown in different concentrations of Hg Cl.sub.2. Control cells do not grow even at concentrations less than 25 .mu.M Hg Cl.sub.2 but the transformed cells grow well even at 100 .mu.M HgCl.sub.2-(FIG. 10). The ability to grow at these high concentrations of mercury in which control is not able to grow, confirms the functionality of both enzymes. Control and transformed clones are grown in LB with 500 .mu.g / ml of spectinomycin for 24 hours at 37.degree. C. Whe...
example 3
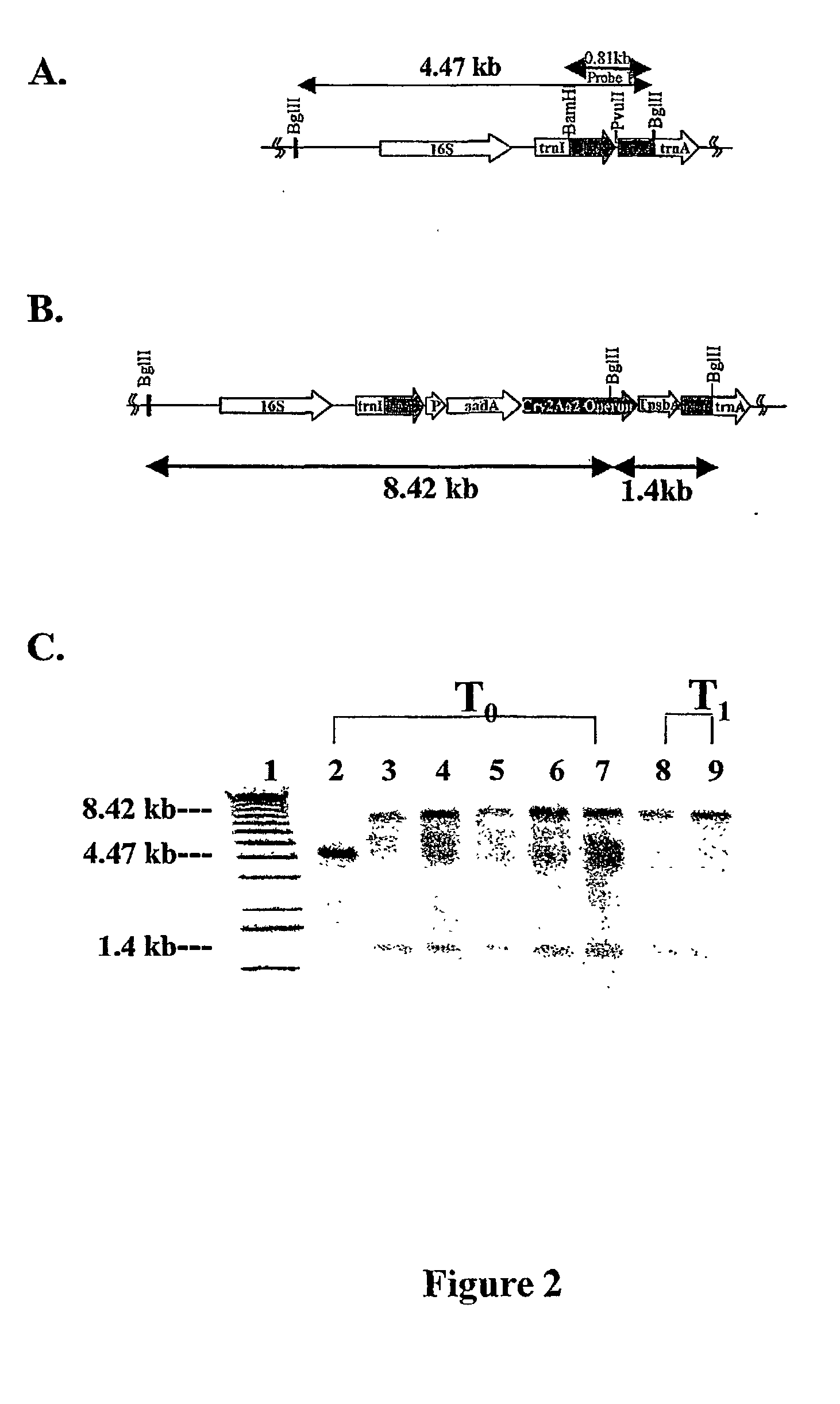
[0133] Chlorella vulgaris transformation vector: The region 16S to 23S of the Chlorella vulgaris chloroplast genome is amplified by PCR using specific primers complementary to rrn16 and to rrn23. The PCR product will be cloned into pCR 2.1 vector available from Promega. The PCR product 16S to 23S is removed from the pCR2.1 vector by a blunt end restriction endonuclease and cloned into the pUC19 in which the multiple cloning site has been removed using a blunt end restriction enzyme (Pvu1I). Then the cassette containing the promoter, the antibiotic resistance gene and the merAB genes is inserted into the new vector (Chlorella transformation vector) using a blunt end restriction enzyme (HincII) that is present in the spacer region between trnA and trnT. The final construct is used for the transformation of Chlorella vulgaris (FIG. 12).
[0134] Bombardment and transformation of Chlorella vulgaris: The biolistic transformation method (Sanford et al. 1993) is optimized for transformation o...
example 4
[0138] Synechocystis transformation vector: The region 16S to 23S of the Synechocystis genome is amplified by PCR using specific primers complementary to rrn 116 and to rrn23. The PCR product is cloned into the pCR 2.1 vector available from Promega. The PCR product 16S to 23S is removed from the pCR2.1 vector by a blunt end restriction endonuclease and cloned into pUC19 in which the multiple cloning site has been removed using a blunt end restriction enzyme (Pvu1I). Then the cassette containing the promoter, the antibiotic resistance gene and the merAB genes is inserted into the new vector (Synechocystis transformation vector) using a blunt end restriction enzyme (HincII) that is present in the spacer region between trnI and trnA. The final construct will be used for the transformation of Synechocystis (FIG. 13).
[0139] Transformation of Synechocystis: A fresh culture of wild type in BG-11 (heterotrophic medium) plus glucose is grown to OD.sub.730=0.5 after 2-3 days of culture. Cells...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical resistance | aaaaa | aaaaa |
| Ratio | aaaaa | aaaaa |
| Stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com