Patents
Literature
132 results about "Immune Precipitates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
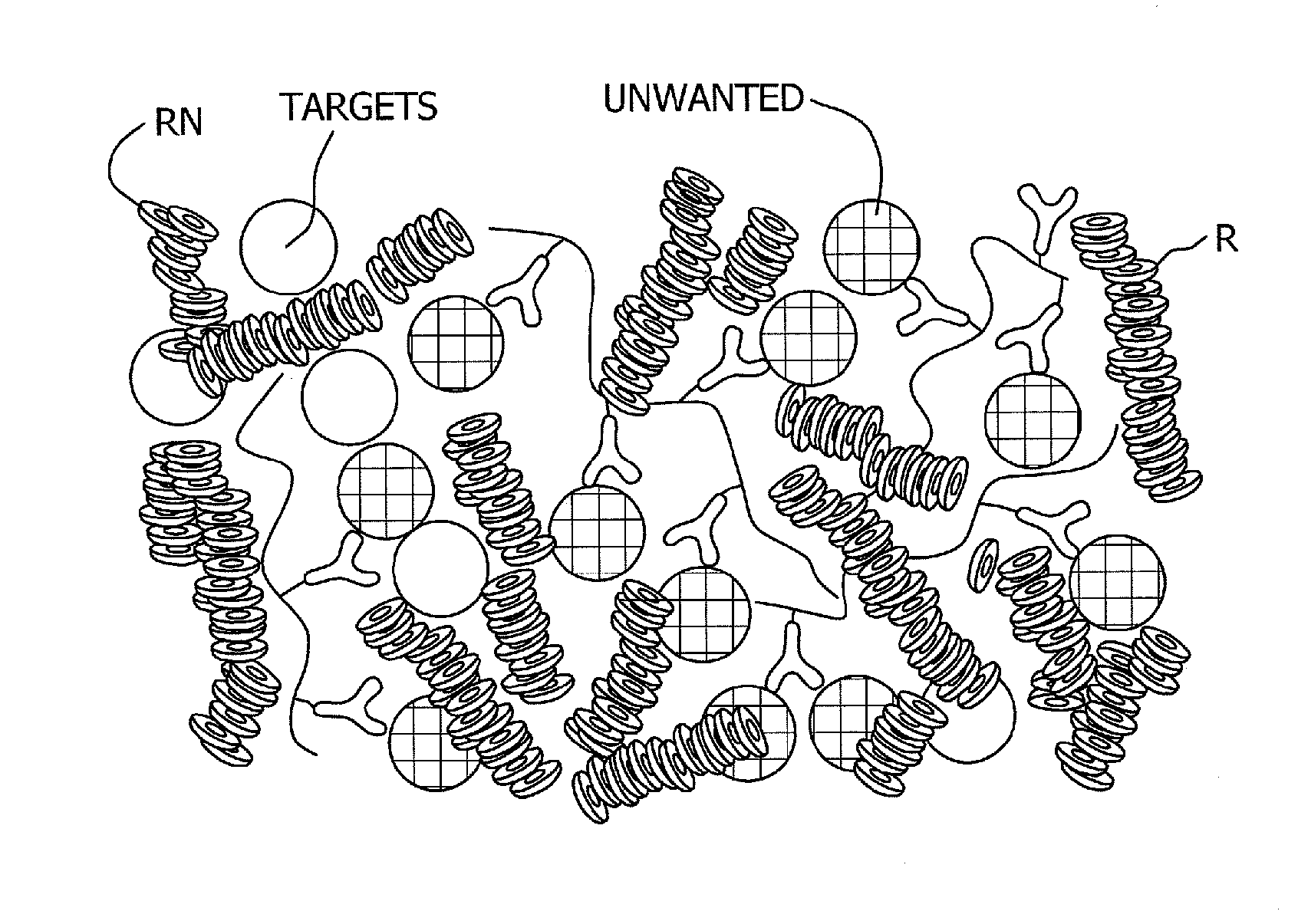
Immunoprecipitation (IP) is the technique of precipitating a protein antigen out of solution using an antibody that specifically binds to that particular protein. This process can be used to isolate and concentrate a particular protein from a sample containing many thousands of different proteins.
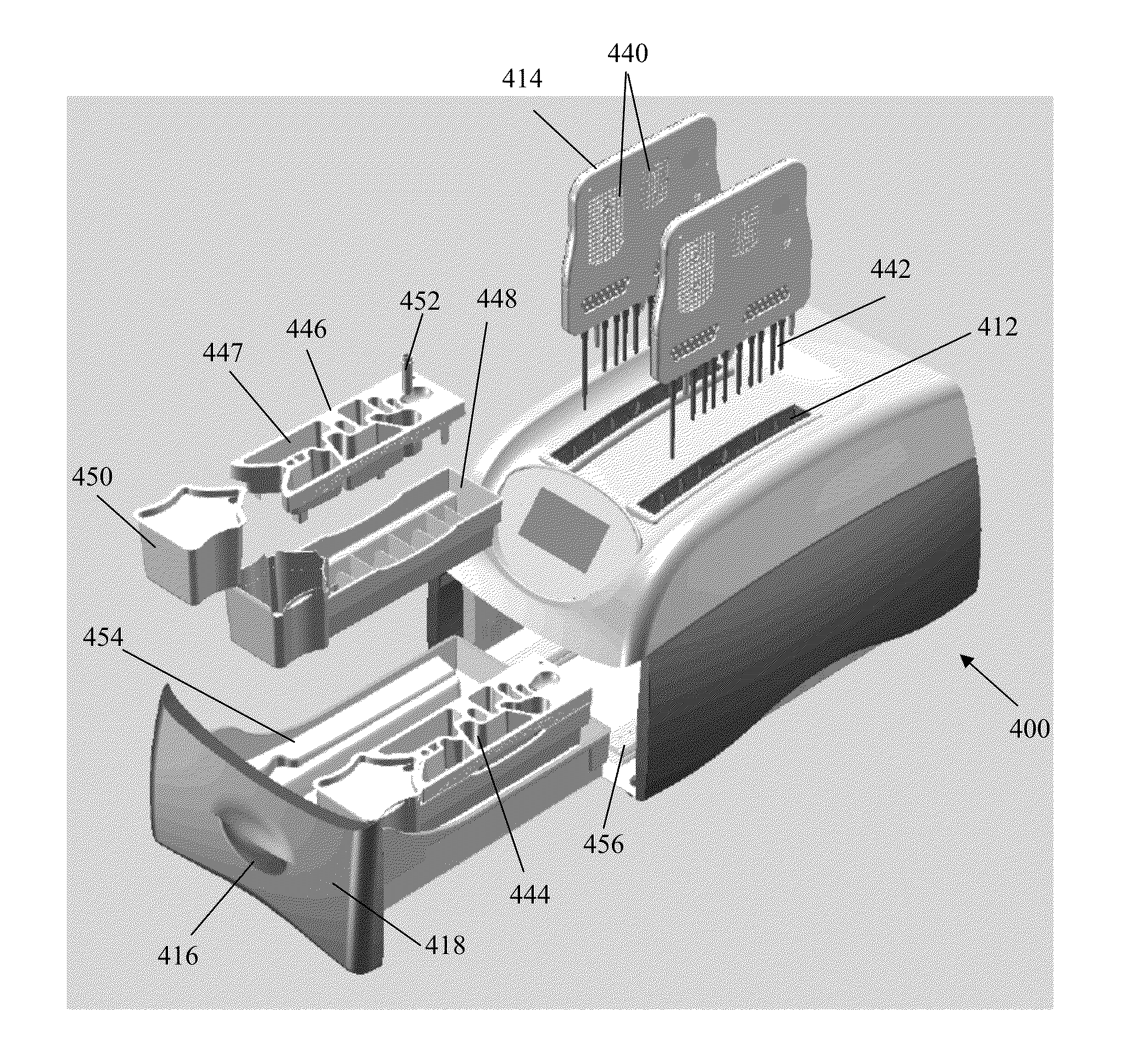
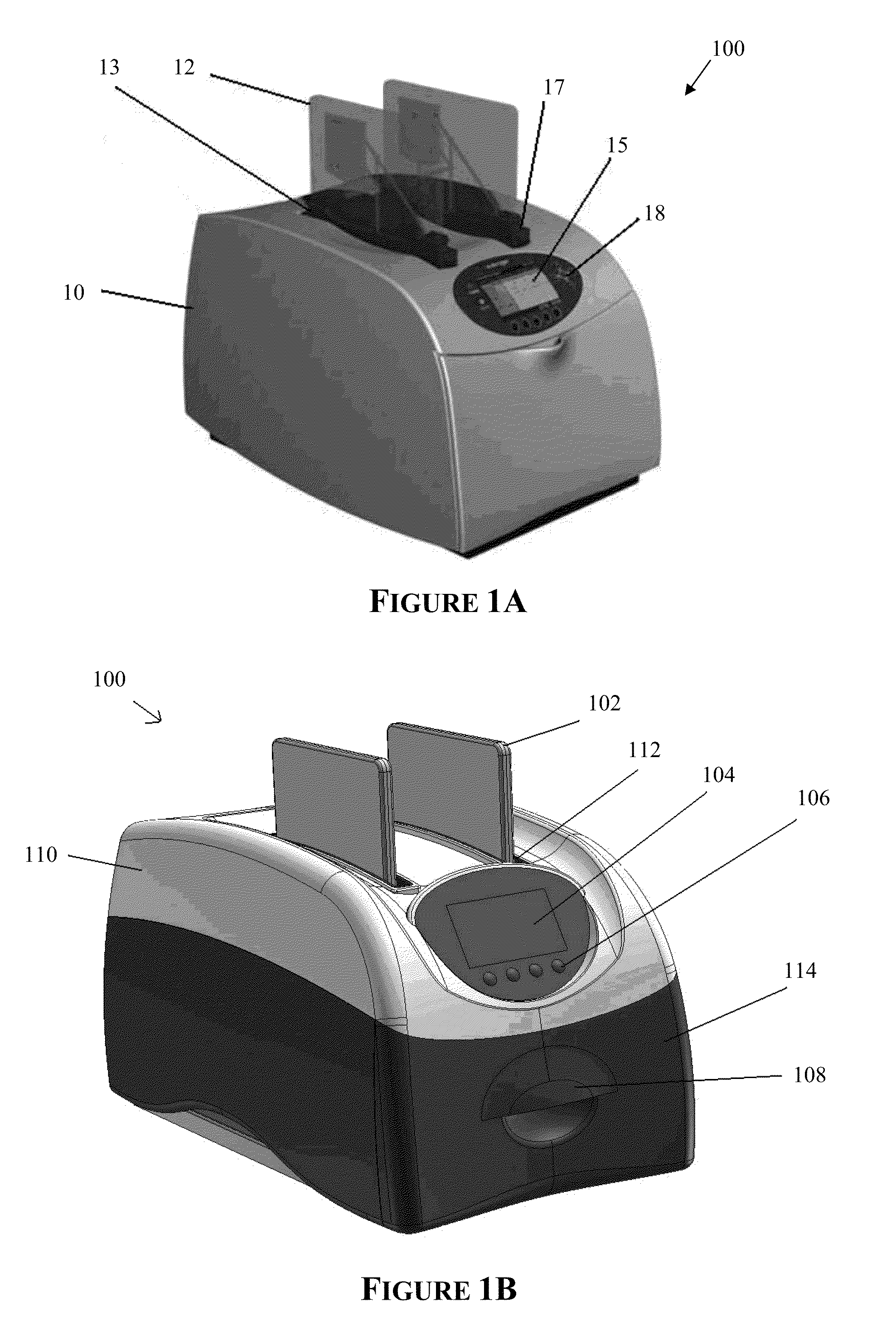
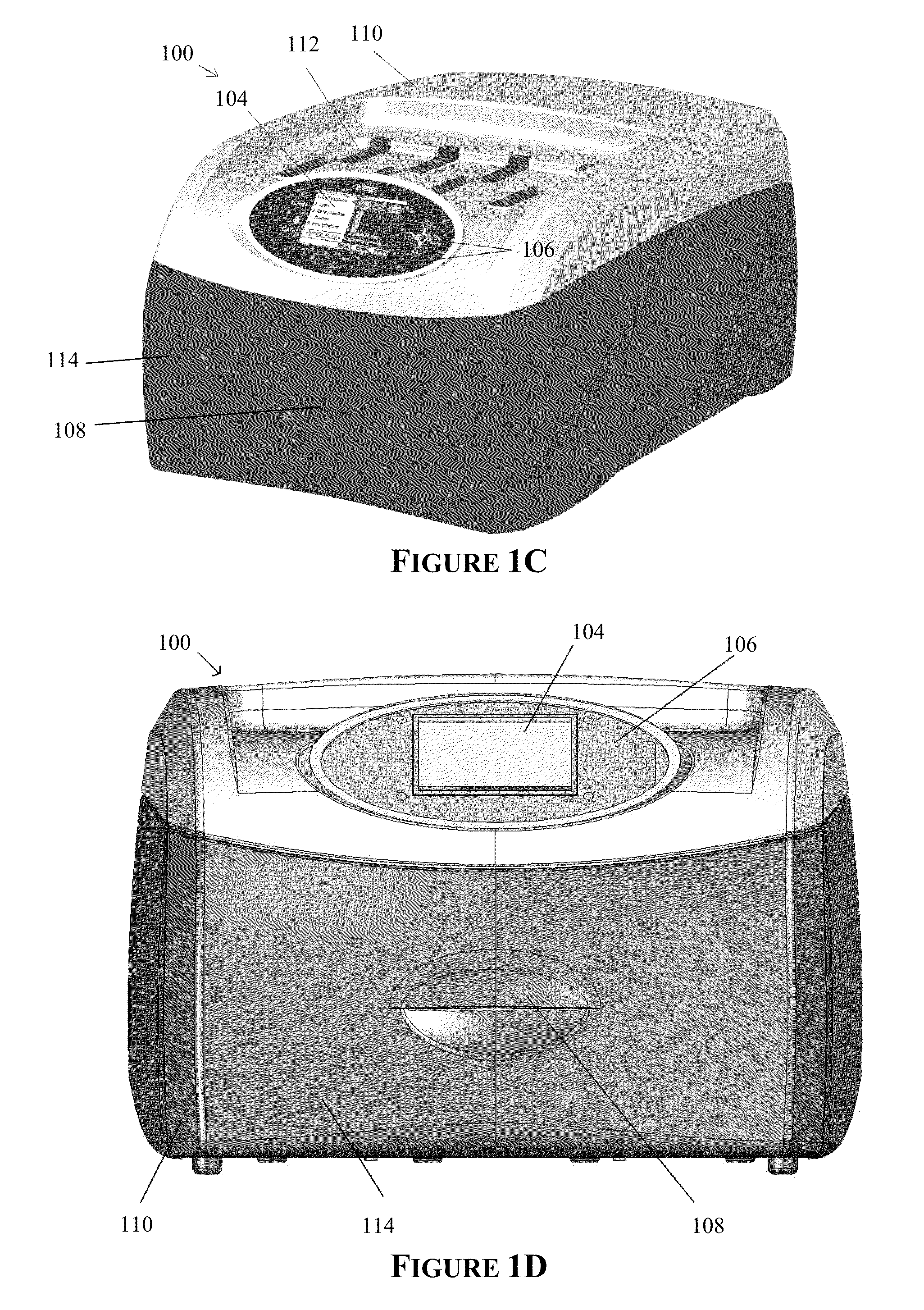
Apparatus for and method of processing biological samples
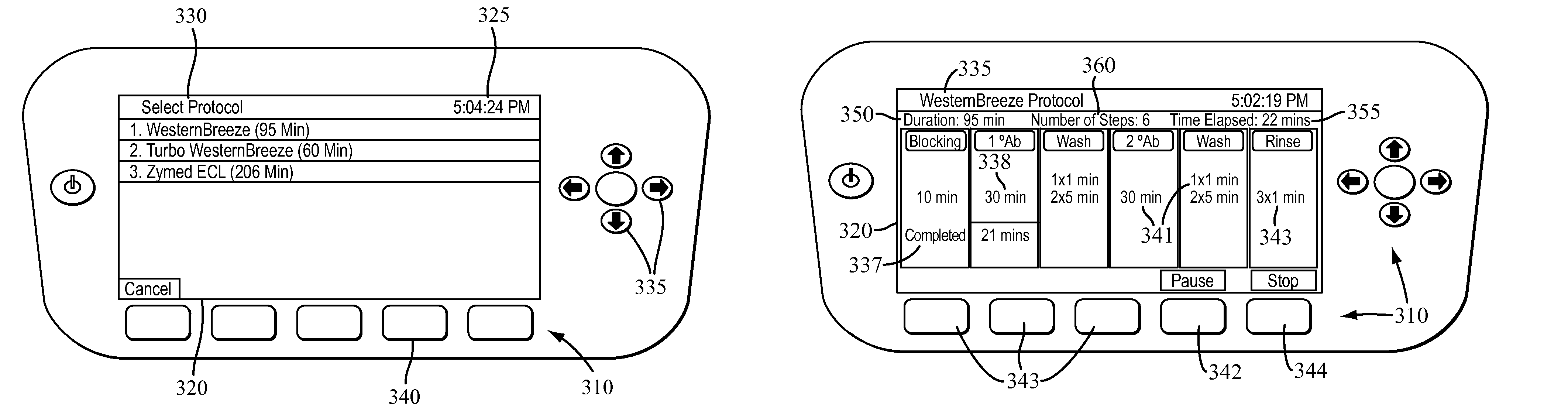
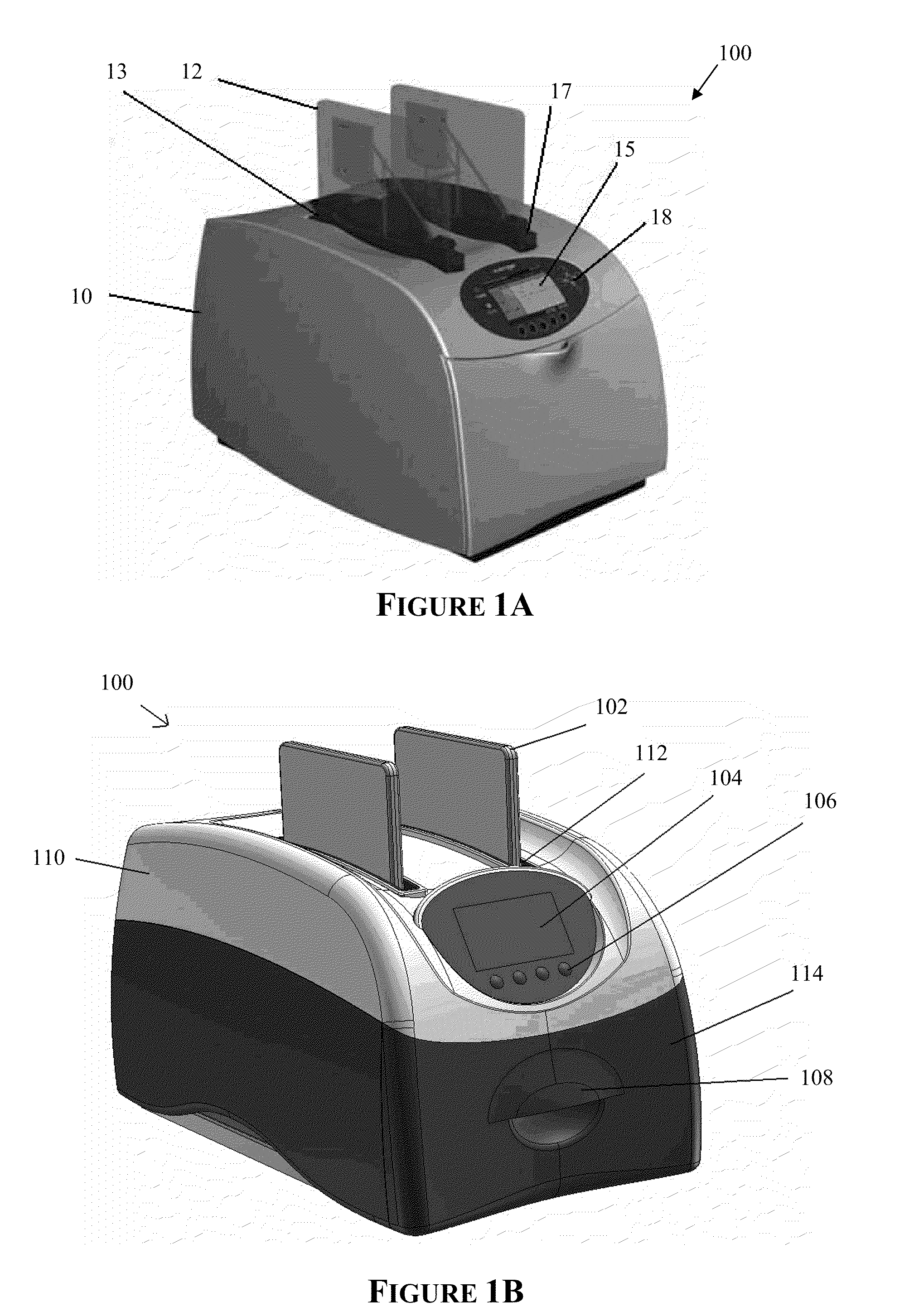
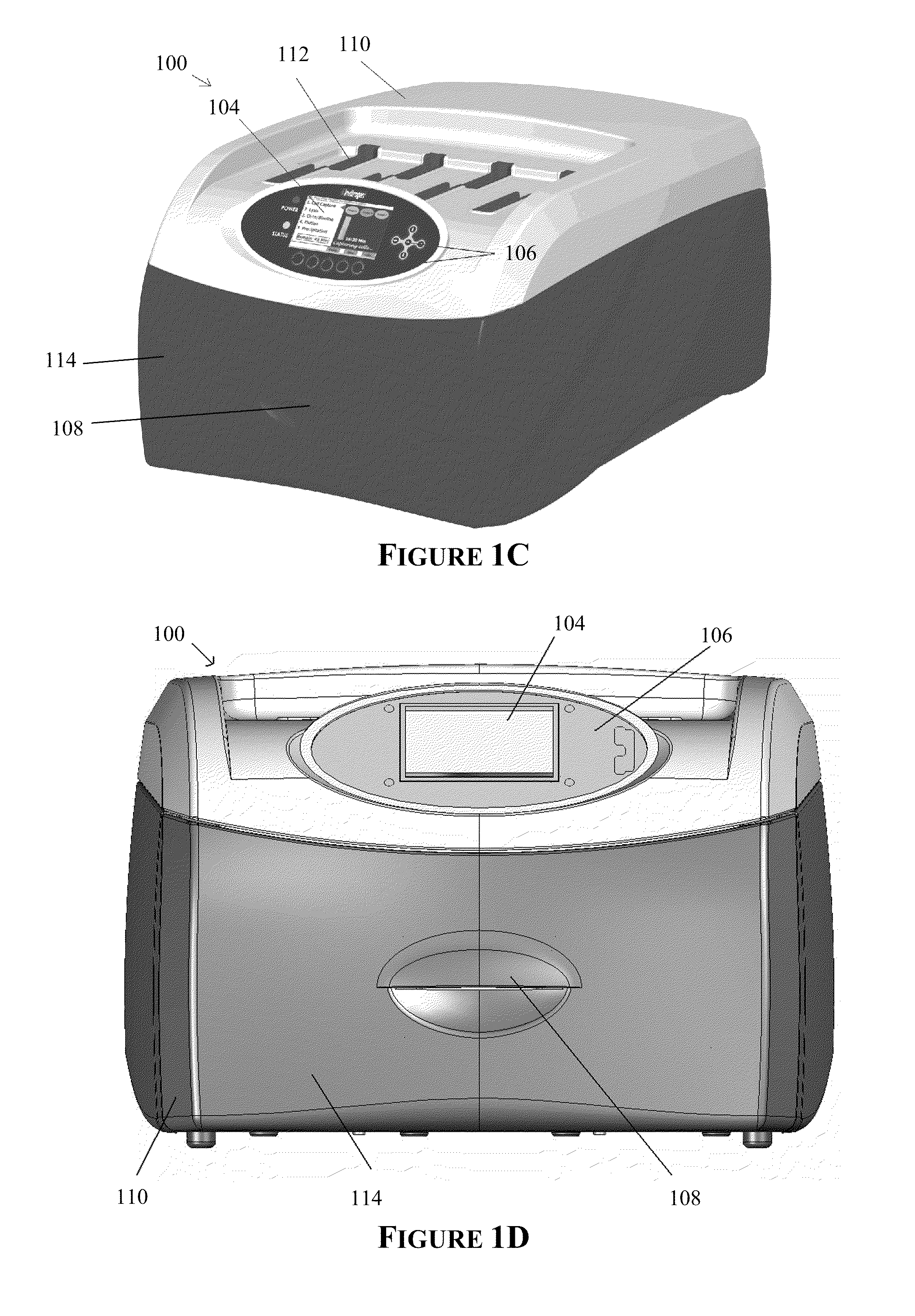
The present invention provides systems, devices, apparatuses and methods for automated bioprocessing. Examples of protocols and bioprocessing procedures suitable for the present invention include but are not limited to: immunoprecipitation, chromatin immunoprecipitation, recombinant protein isolation, nucleic acid separation and isolation, protein labeling, separation and isolation, cell separation and isolation, food safety analysis and automatic bead based separation. In some embodiments, the invention provides automated systems, automated devices, automated cartridges and automated methods of western blot processing. Other embodiments include automated systems, automated devices, automated cartridges and automated methods for separation, preparation and purification of nucleic acids, such as DNA or RNA or fragments thereof, including plasmid DNA, genomic DNA, bacterial DNA, viral DNA and any other DNA, and for automated systems, automated devices, automated cartridges and automated methods for processing, separation and purification of proteins, peptides and the like.
Owner:LIFE TECH CORP
Rapid way to obtain high expression clones of mammalian cells using a methylcellulose and immunoprecipitation screening method
InactiveUS20050118652A1High throughput screeningImmunoglobulins against cytokines/lymphokines/interferonsPeptide preparation methodsHigh-Throughput Screening MethodsScreening method
The invention provides a genetic screening method for identifying a transfected cell expressing the polypeptide of interest. The methods allows for high throughput screening of recombinant cells for elevated levels of expression of the polypeptide of interest. The invention also provides capture media, formulations and methods of making and using thereof.
Owner:JANSSEN BIOTECH INC
Method of identifying active chromatin domains
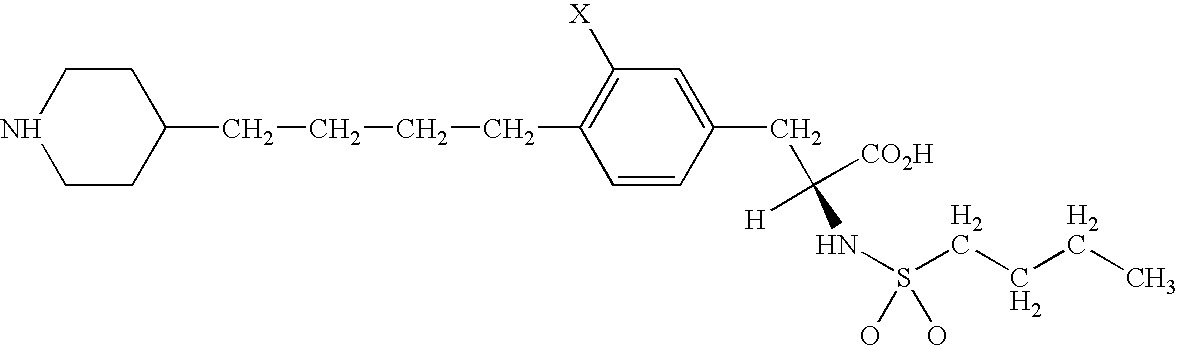
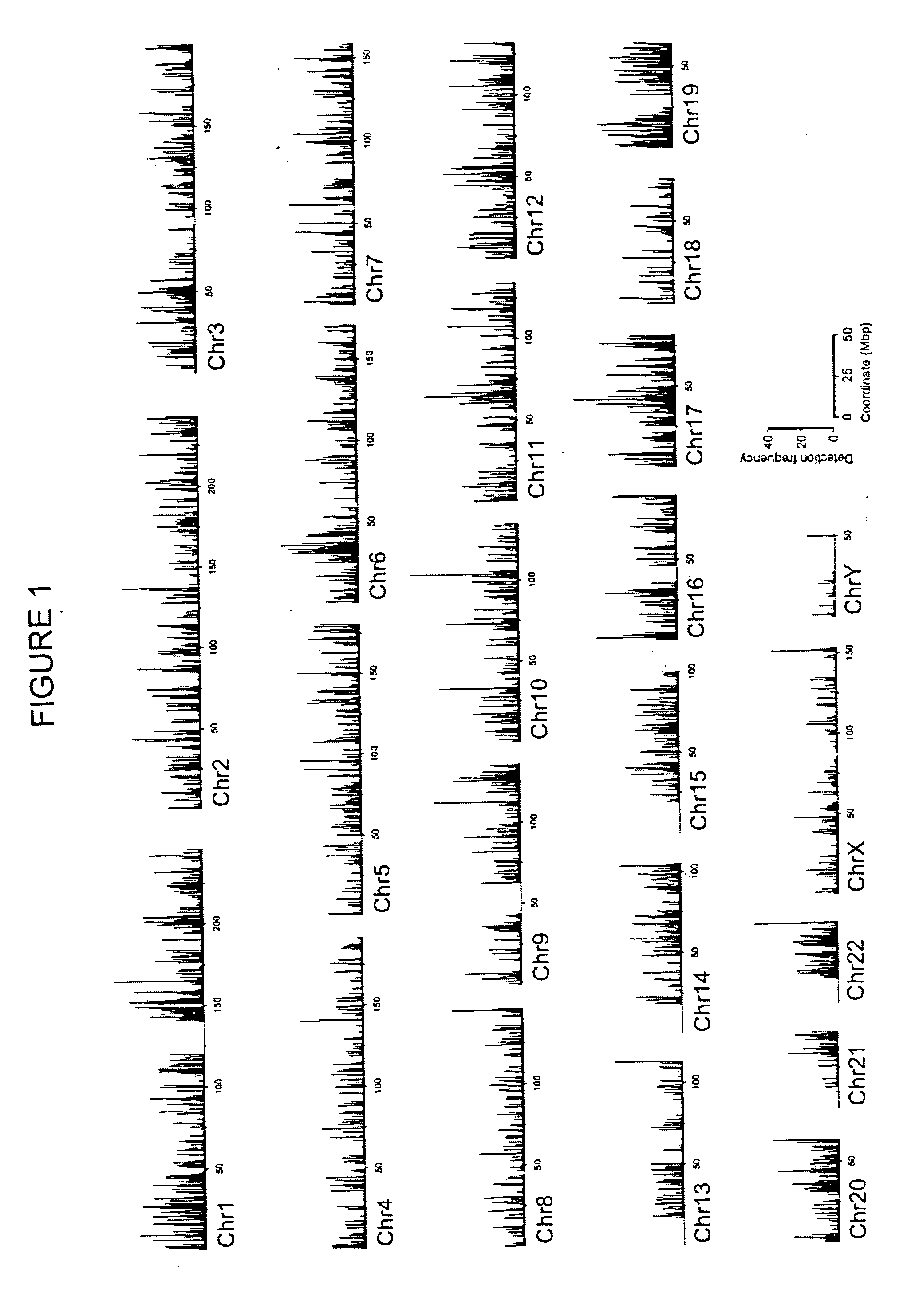
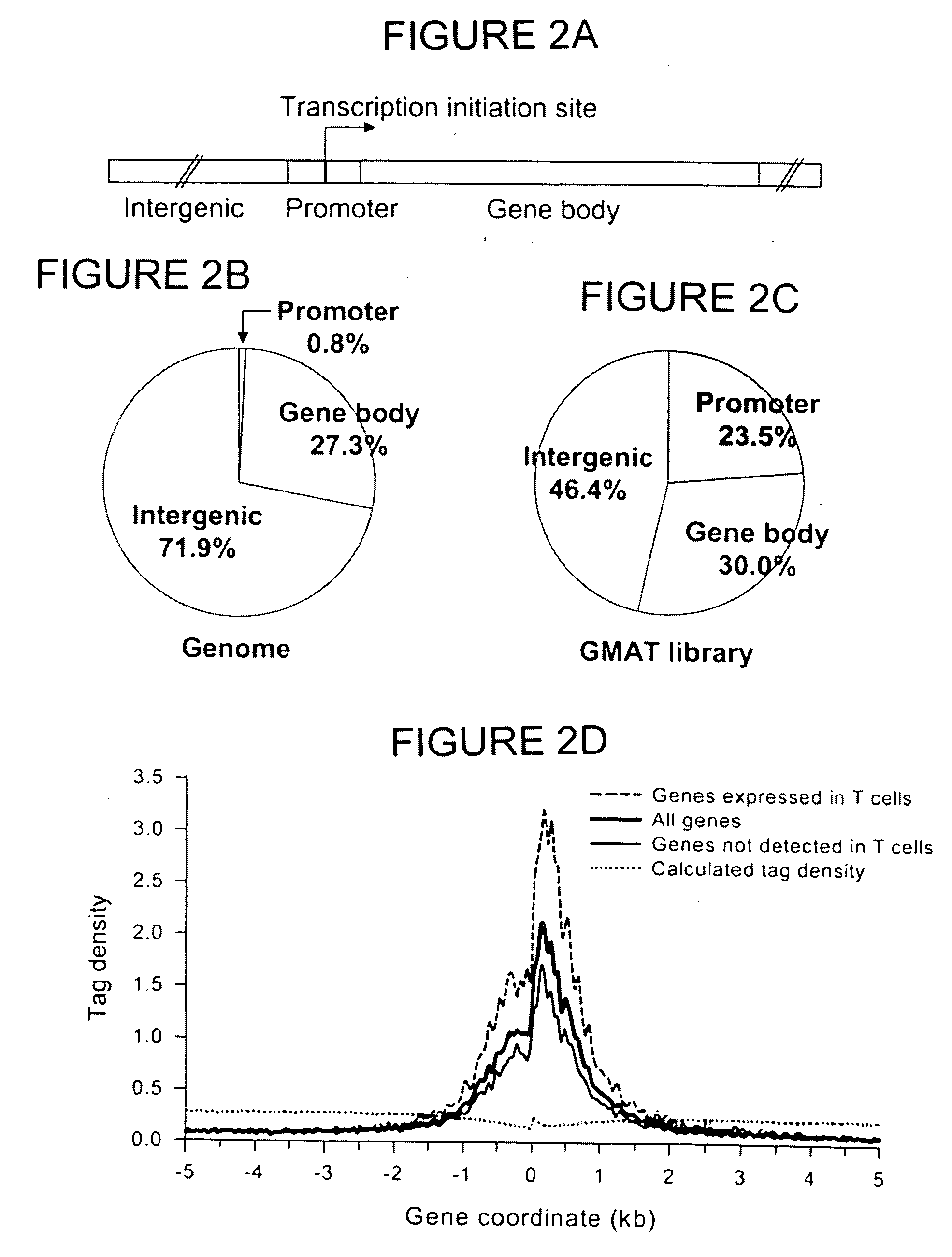
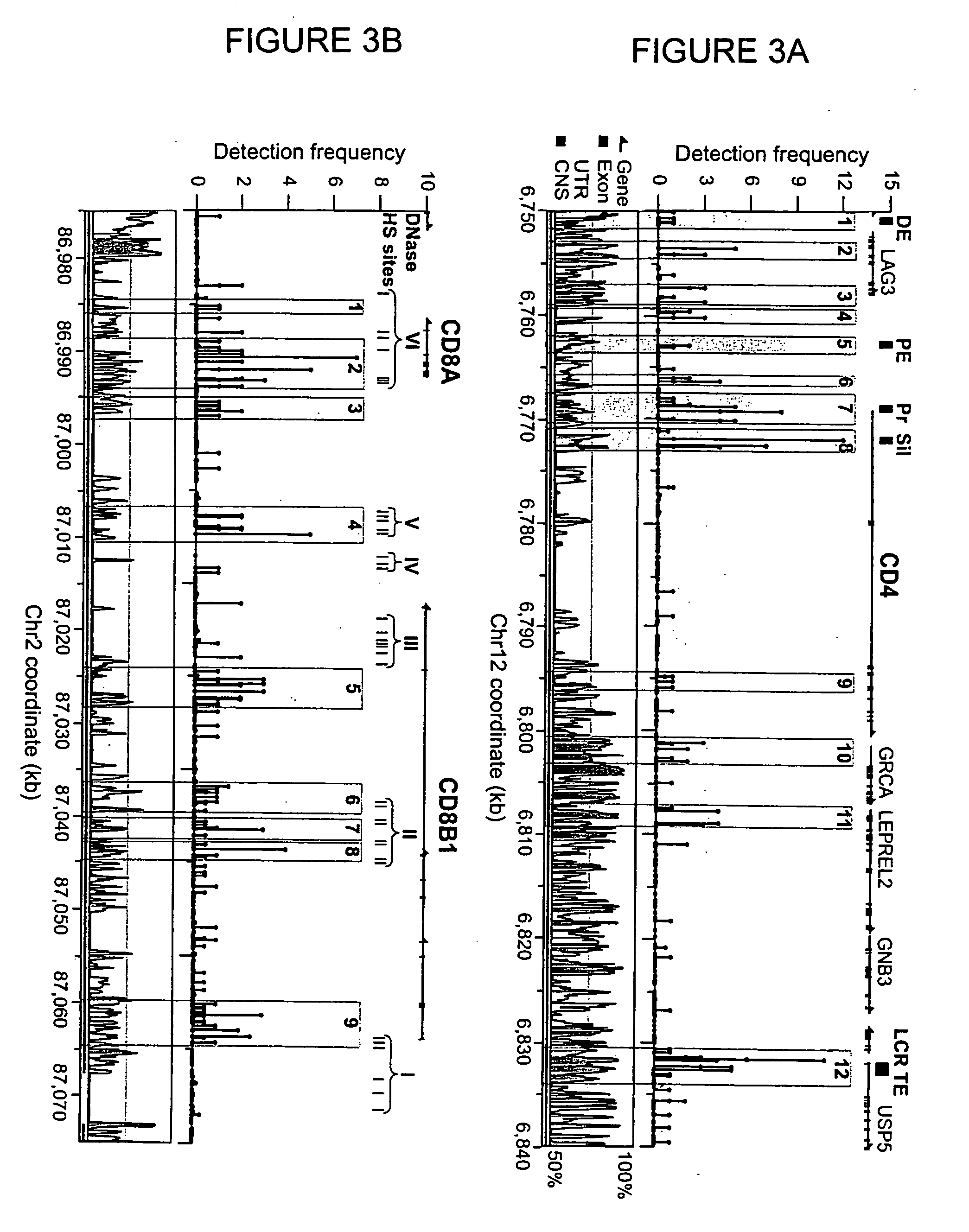
The invention provides a method of mapping DNA-protein interactions within a genome by fixing living cells to cross-link DNA and proteins, lysing the cells, and isolating chromatin by immunoprecipitation. DNA is purified and a SAGE protocol is performed on the purified DNA to produce GMAT-tag sequences, which are compared to a genomic sequence of the living cells to map DNA-protein interactions. The invention further provides a method of identifying an active chromatin domain and a method of identifying aberrant chromatin acetylation, wherein chromatin immunoprecipitation is performed using an antibody recognizing acetylated histone protein.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
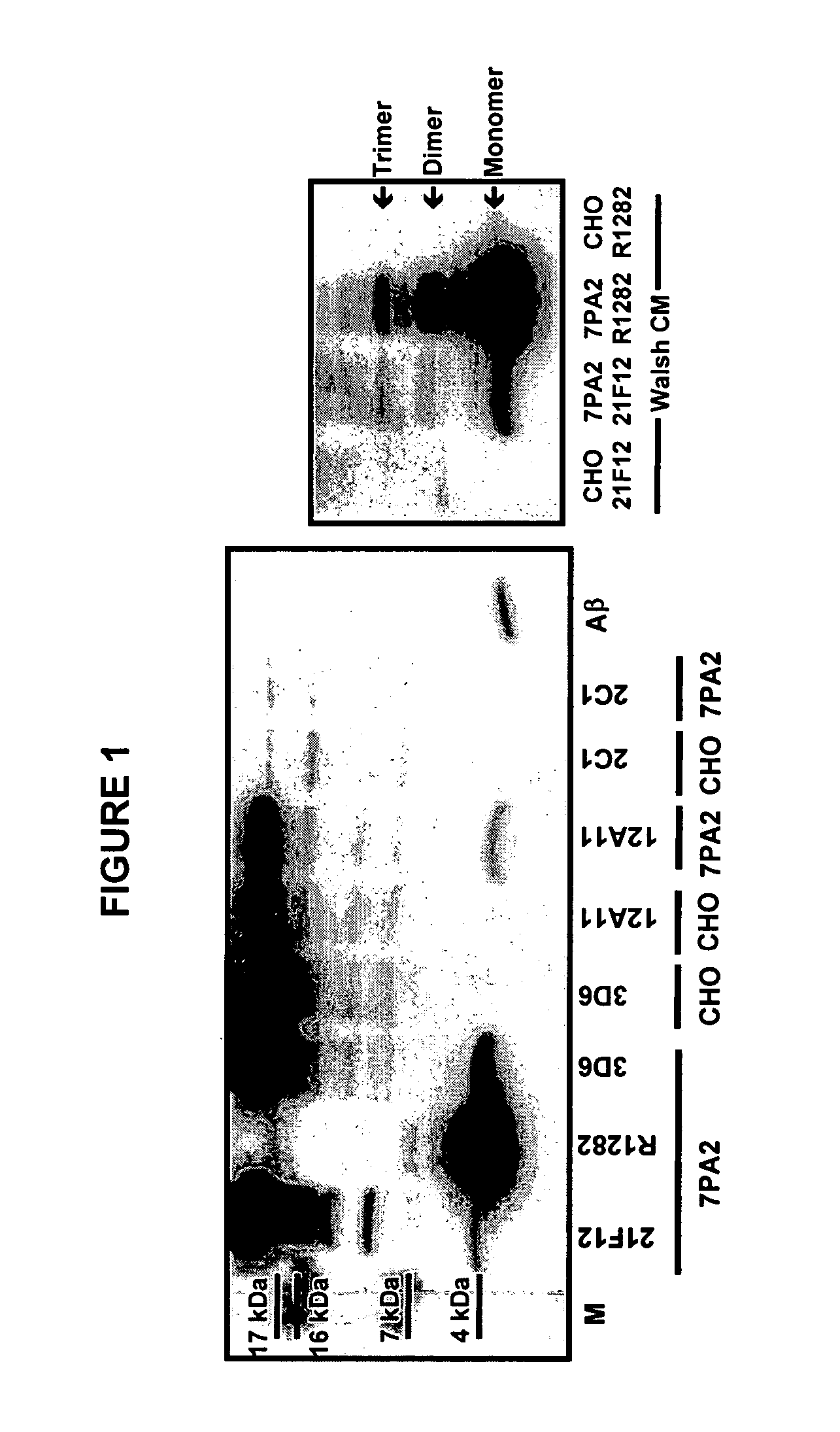
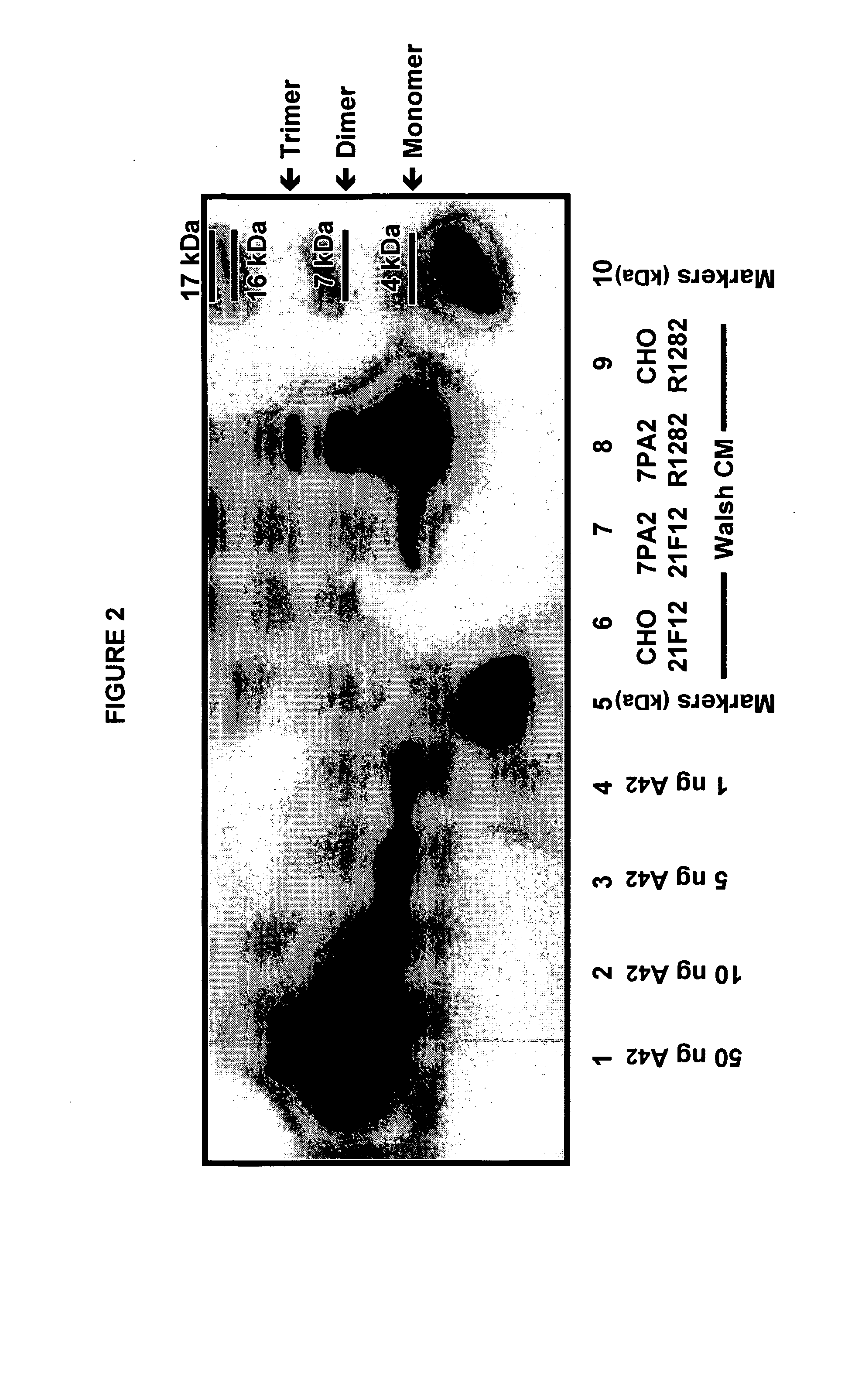
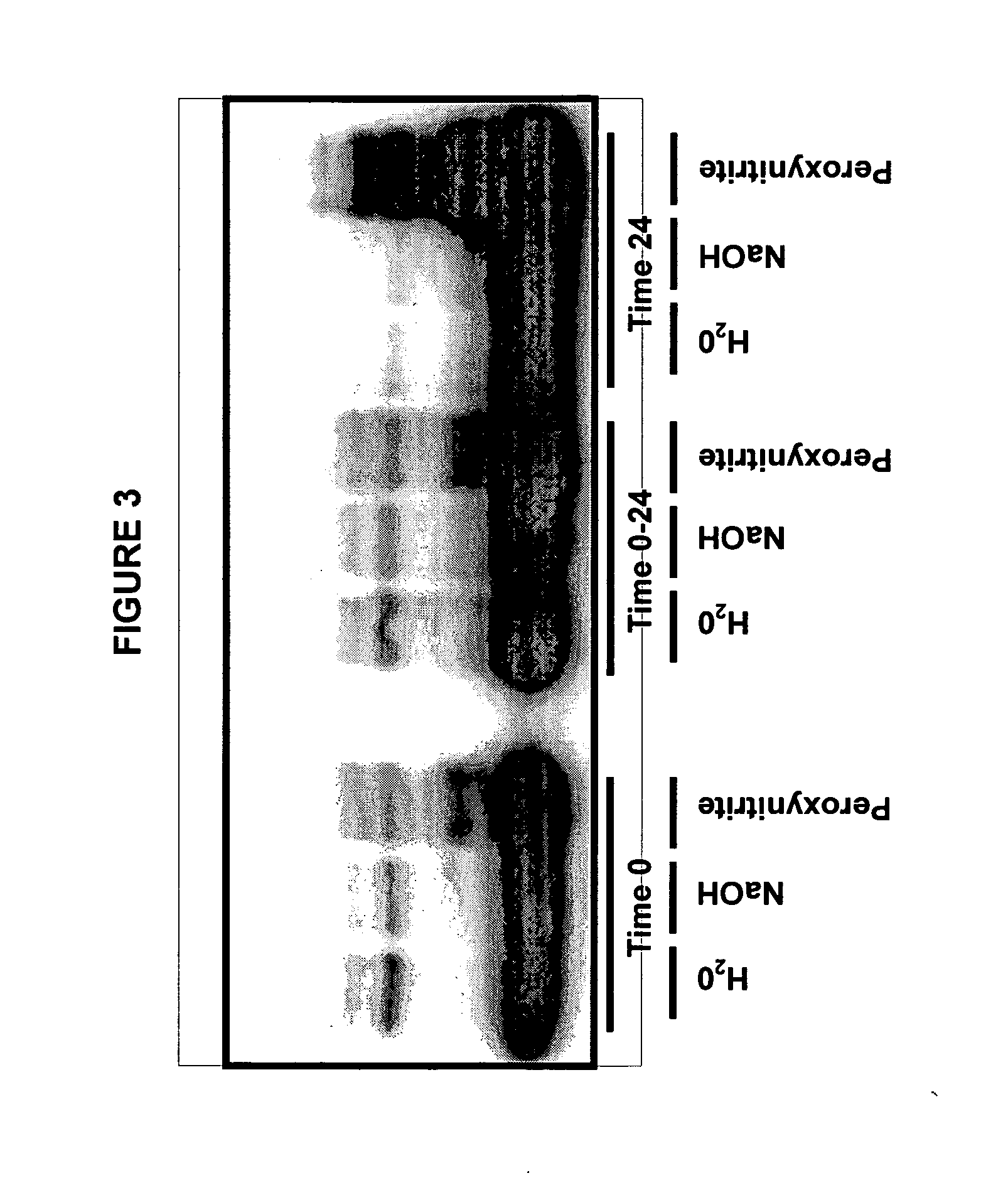
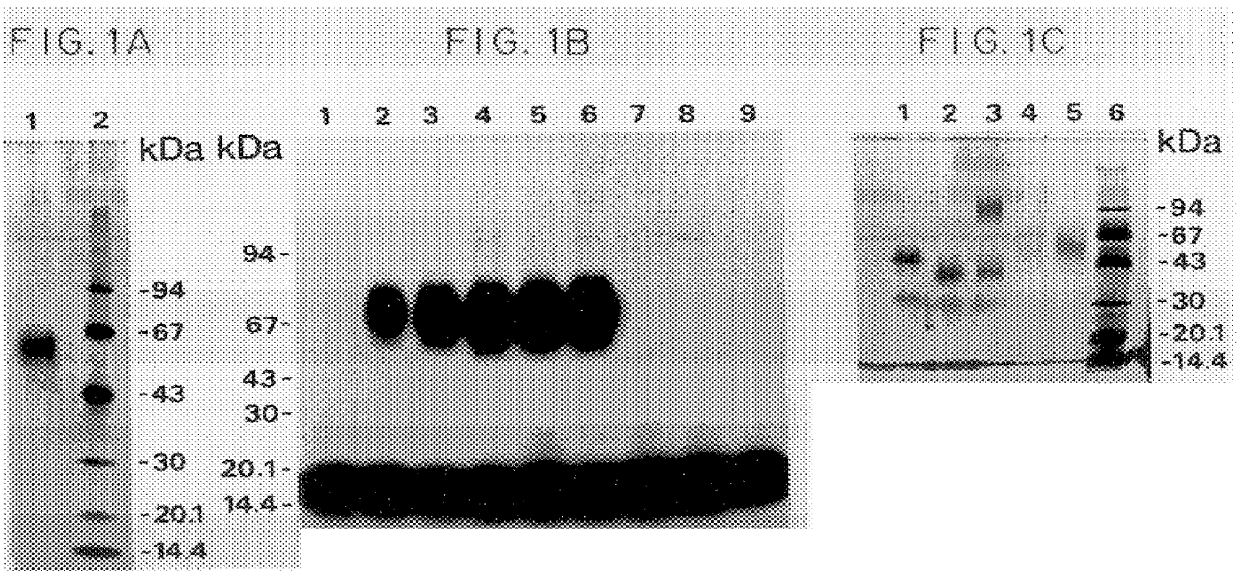
Immunoprecipitation-based assay for predicting in vivo efficacy of beta-amyloid antibodies
InactiveUS20060240486A1Rapid improvement in cognitionImprove bindingCompound screeningApoptosis detectionAβ oligomersAmyloid
In various aspects, the present invention provides methods and kits for predicting the therapeutic efficacy of an immunological reagent, identifying an immunological reagent having therapeutic efficacy, or both, for the treatment of an amyloidogenic disorder by comparing the amount of Aβ monomer in an Aβ preparation which binds to the immunological reagent to an amount of one or more Aβ oligomers in the Aβ preparation which bind to the immunological reagent to determine a relative bound amount, and predicting the efficacy of the immunological reagent, identifying an immunological reagent having therapeutic efficacy, or both, for the treatment of an amyloidogenic disorder based at least on the relative bound amount.
Owner:JANSSEN ALZHEIMER IMMUNOTHERAPY +1
Antibodies and their use
InactiveUS6113897APeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadUrokinase Plasminogen Activator
A monoclonal or polyclonal antibody directed against urokinase plasminogen activator receptor (u-PAR), or a subsequence, analogue or glycosylation variant thereof. Antibodies are disclosed which react with free u-PAR or with complexes between u-PA and u-PAR and which are capable of 1) catching u-PAR in ELISA, or 2) detecting u-PAR, e.g. in blotting, or 3) in radioimmunoprecipitation assay precipitate purified u-PAR in intact or fragment form, or 4) is useful for immunohistochemical detection of u-PAR, e.g. in immunostaining of cancer cells, such as in tissue sections at the invasive front, or 5) inhibits the binding of pro-u-PA and active u-PA and thereby inhibits cell surface plasminogen activation. Methods are disclosed 1) for detecting or quantifying u-PAR, 2) for targeting a diagnostic to a cell containing a u-PAR on the surface, 3) for preventing or counteracting proteolytic activity in a mammal. Methods for for selecting a substance suitable for inhibiting u-PA / u-PAR interaction, for preventing or counteracting localized proteolytical activity in a mammal, for inhibiting the invasion and / or metastasis comprise the use of the antibodies and of nude mice inoculated with human cancer cells which are known to invade and / or metastasize in mice and having a distinct color, f.x. obtained by means of an enzyme and a chromogenic substrate for the enzyme, the color being different from the cells of the mouse.
Owner:CANCERFORSKNINGSFONDEN AF 1989 FONDEN TIL FREMME
Antibodies binding a gamma carboxyglutamic acid displaying epitope
Antibodies are described, which are suitable for identification of gamma-carboxyglutamic acid (Gla), said antibodies having an ability of identifying Gla residues in proteins and / or peptides, while not reacting with glutamic (Glu) residues in corresponding proteins and / or peptides. Methods for the preparation and identification of said antibodies as well as methods for detection of Gla in biological fluids, tissue extracts, tissue specimens, in which methods said antibodies are used, are also described. There is also described the use of said antibodies for immunopurification of Gla-containing proteins or peptides by, for instance, immunoprecipitation or immunoaffinity chromatography.
Owner:BIOMEDICA DIAGNOSTICS INC +1
Compositions and methods for regulating sas1r
InactiveUS20100183617A1Preserve ovarian reserveCompound screeningApoptosis detectionSperm proteinEmbryo
The present invention provides compositions and methods useful for regulating fertilization and for use as a contraceptive based on the discovery herein of an oocyte specific protein, SAS1R (Sperm Acrosomal SLLP1 Receptor), which is a sperm protein receptor. Six SAS1R variants, including the full length SAS1R, were identified. mSLLP1 and SAS1R co-localized to oocytes and to acrosomes of acrosome-reacted sperm. Interactions between mSLLP1 and SAS1R were demonstrated by far-western analysis, in a yeast two-hybrid system under stringent selection conditions, and by immunoprecipitation of SAS1R by anti-mSLLP1 as well as the converse. Purified recombinant SAS1R was found to have protease activity, to inhibit fertilization in-vitro, and to induce an immune response in females. Together, the results suggest SAS1R is a proteolytically active, oocyte and early embryo specific oolemmal metalloprotease receptor for the sperm intra-acrosomal ligand SLLP1 and is a target for regulating fertilization and as a contraceptive.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
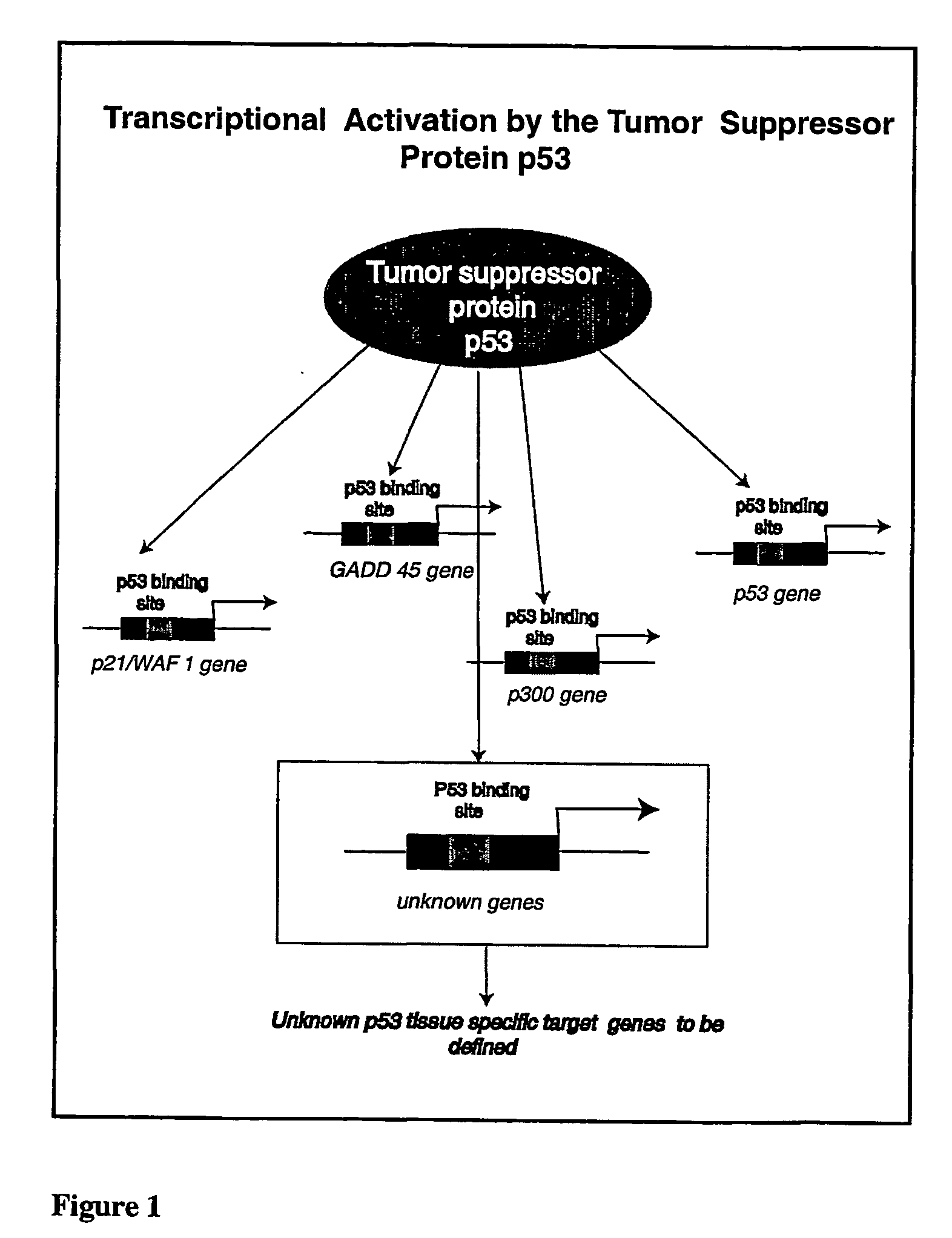
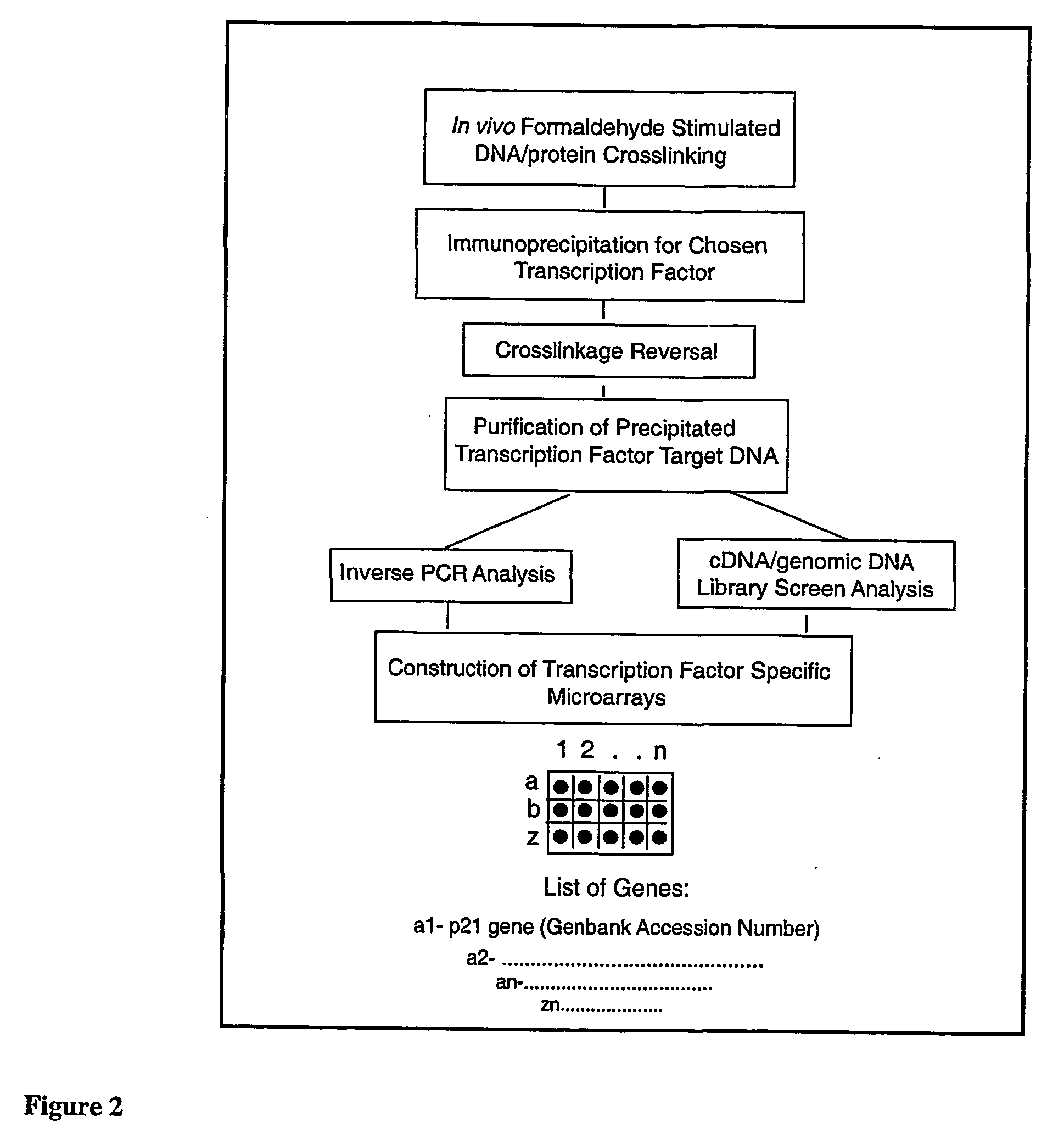
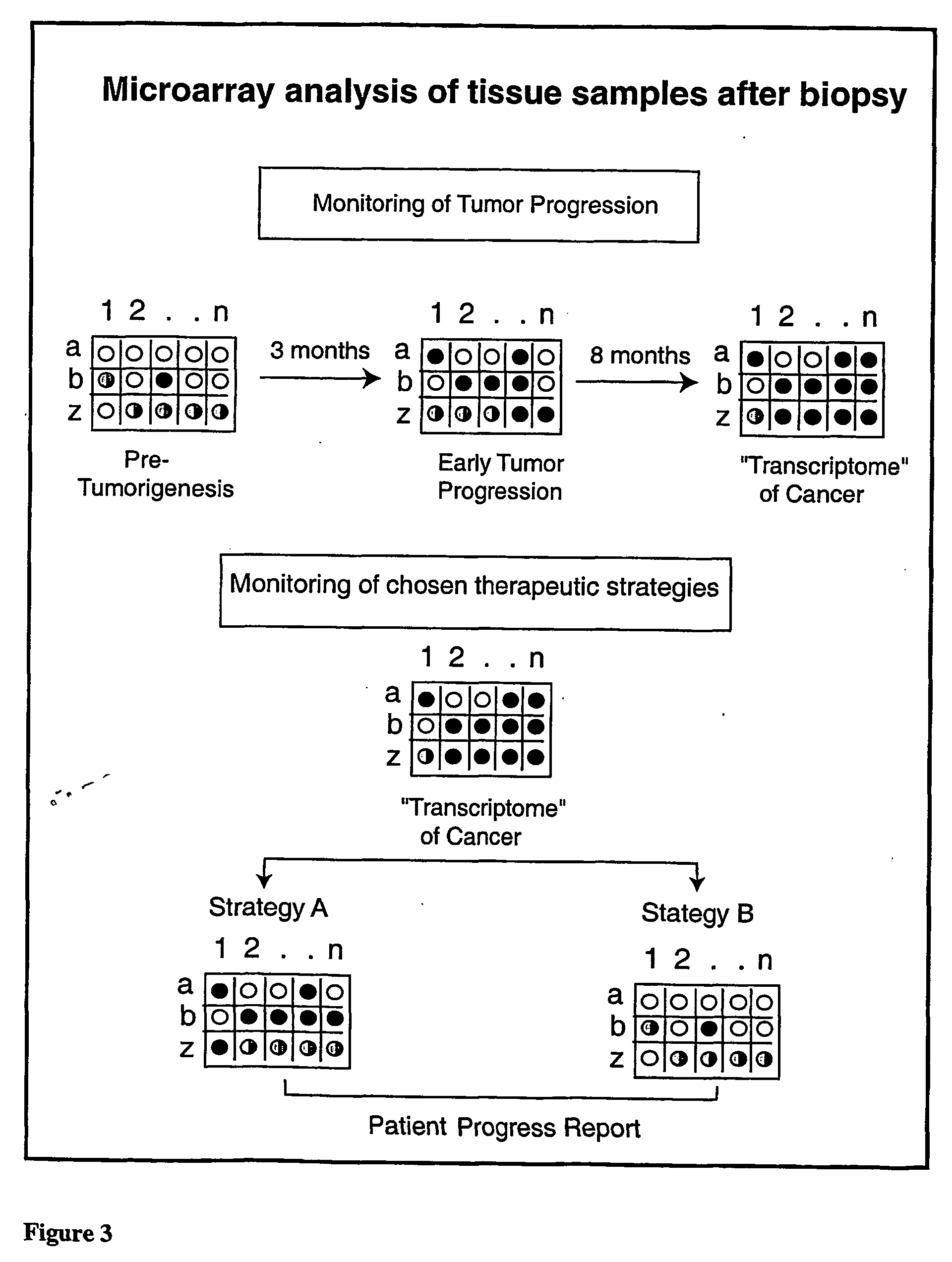
Micro-arrayed organization of transcription factor target genes
InactiveUS20050079492A1Decreasing acquisitionDecreased background random sequenceMicrobiological testing/measurementDNA preparationProtein targetImmunoprecipitation
The following invention outlines methodologies for the construction and utilization of transcription factor direct target gene microarrays of both DNA and corresponding protein / peptide target origin. The technology entails the array / microarray annotation and organization of transcription factor direct loci and corresponding protein products identified through modified and improved versions of chromosomal immunoprecipitation (CHIP) and molecular cloning procedures. It allows for the formulation of physiologically directed arrays which result in a thorough, focused characterization of the genetic and biochemical regulation occurring within a give population of cells or a given tissue. Arrays and microarrays of direct targets for any given transcription factor created utilizing this technology are substantially more clinically relevant for purposes of medical diagnostics and patient prognostics than conventional microarrays due to the physiologically focused nature and the transcription factor targets. In addition, the characterization and array organization of transcription factor target protein products and the assessment of their interactions with other proteins and / or small molecules is of critical importance for the purposes of understanding cellular and ultimately the design of therapeutics for human anomalies.
Owner:BURS JR ROBERT M +2
Lyophilized viper antivenin and preparation method thereof
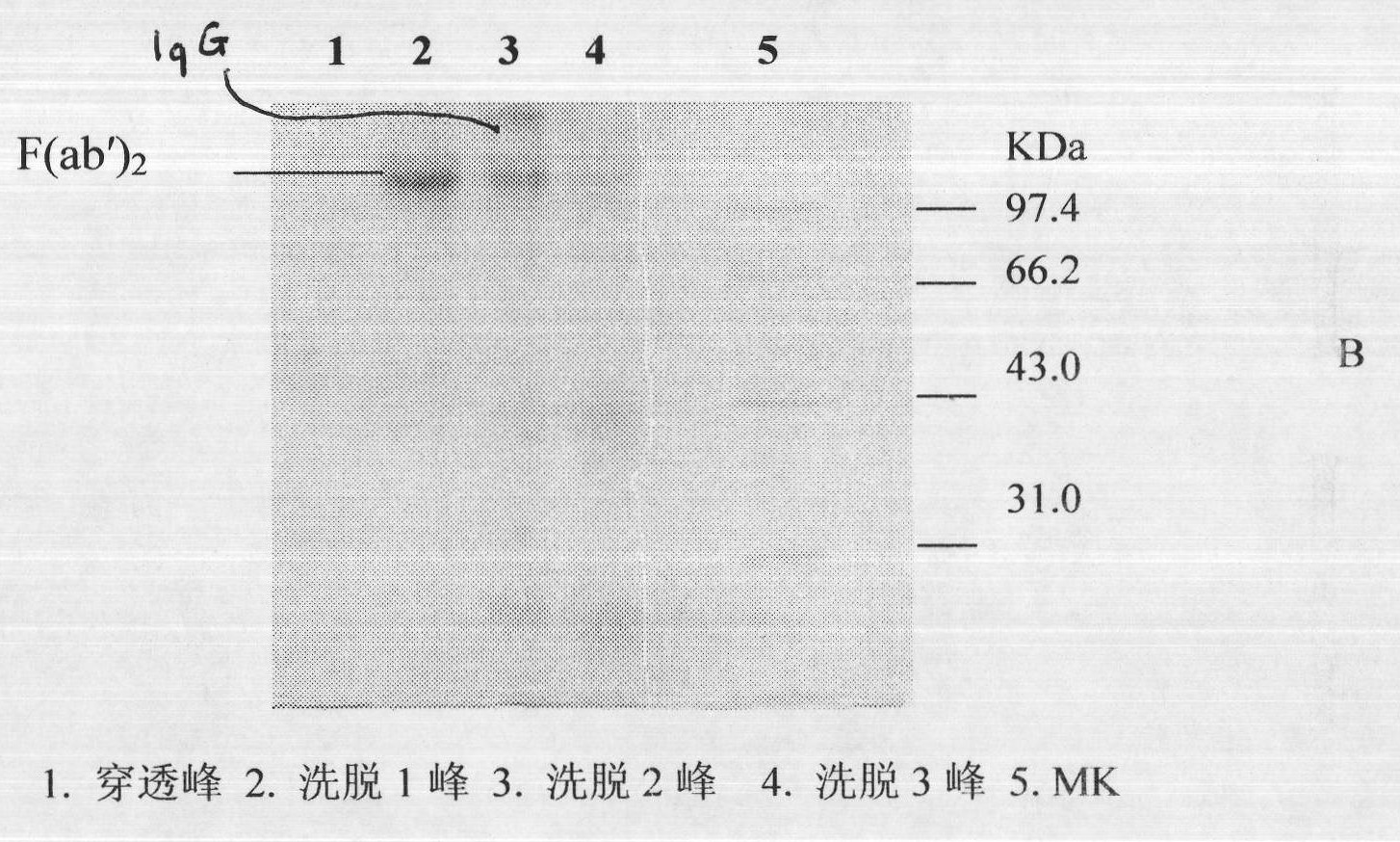
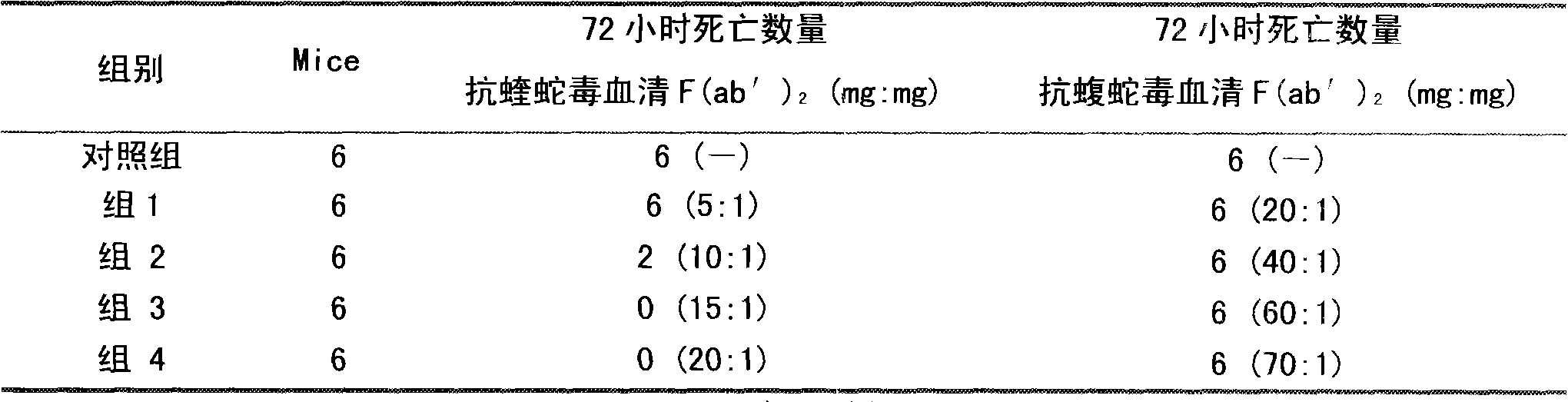
ActiveCN101816789AHigh potencyGuaranteed curative effectAntinoxious agentsMammal material medical ingredientsMass ratioBlood plasma
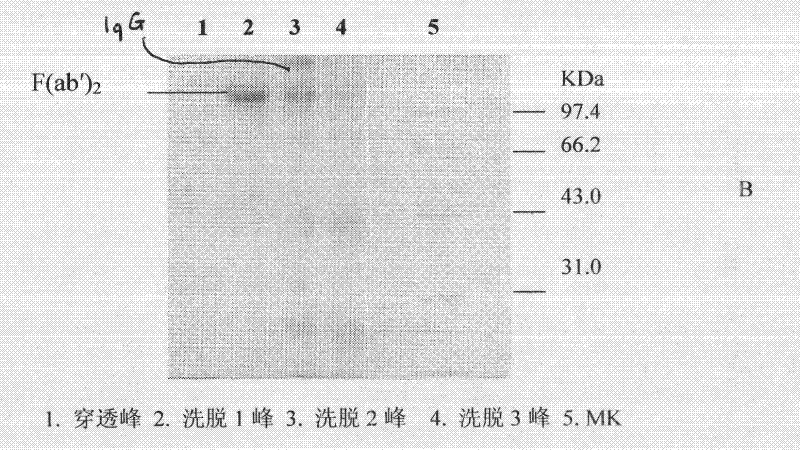
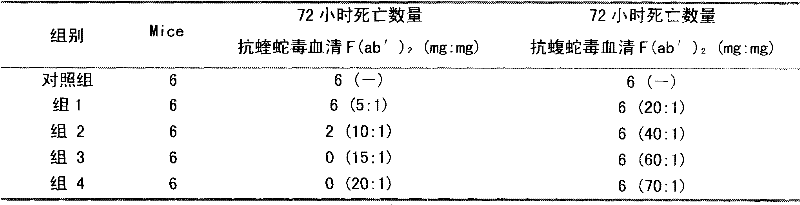
The invention discloses a lyophilized viper antivenin and a preparation method thereof, belonging to biochemical products, more particularly relating to an antivenin and a preparation technology thereof. The mass ratio of the antivenin and viper venom is 15:1, which can specifically neutralize the viper venom injected to mouse; as determined by immunodiffusion, the immunoprecipitation line appears in case that the ratio of viper antivenin F(ab')2 in lyophilized form to viper venom is 8:1; and other detected items conform to the quality standard of antivenin in Chinese Pharmacopoeia 2010. The detection of Phenyl-Sepharose (low-sub) FF column chromatography result shows that: activity is centralized at eluting peak 1, micromolecule impurity proteins are centralized at penetration peal, eluting peak 2 and eluting peak 3. According to the technology of the invention, immune blood plasma is resulted from viper venom immune horse, IgG is prepared by salting out the immune blood plasma, and F(ab')2 active fragment is obtained after the IgG is subject to enzymolysis and purification by a hydrophobic column. The lyophilized viper antivenin has strong specificity, high potency and more than 85% of the purity of antivenin F(ab')2 in lyophilized form.
Owner:浙江健博生物科技股份有限公司
ELISA kit for detecting Salmonella pullorum antibody
ActiveCN103995126AReduce stressImprove featuresBiological material analysisBiological testingAntigenElisa kit
An ELISA kit for detecting a Salmonella pullorum antibody is established by screening Salmonella pullorum dominant antigen GroEL through an immunoprecipitation technology, expressing GroEL recombinant protein through utilizing a prokaryotic expression vector, and utilizing an antigen protein. The kit can reduce the response of chicken in the detection process of the Salmonella pullorum antibody, and can improve the detection specificity and the sensitivity.
Owner:WENS FOOD GRP CO LTD
Anti-human CEACAM5 monoclonal antibody and preparation method and application thereof
ActiveCN108341876AHigh affinityImprove featuresMicroorganism based processesImmunoglobulins against cell receptors/antigens/surface-determinantsStainingImmunoprecipitation
The invention discloses an anti-human CEACAM5 monoclonal antibody and a preparation method and application thereof, and the anti-human CEACAM5 monoclonal antibody is produced by a mouse hybridoma cellline with the accession number CCTCC NO: C2016129. The preparation method comprises the following steps: S1, immunizing a mouse with whole cell protein, lysed by human gastric cancer cells, as an immunogen; S2, fusing spleen cells of the immunized mouse obtained in the step S1 with mouse myeloma cells; S3, selecting a hybridoma cell line stably secreting antibodies after cell cloning occurs during the cell fusing in the step 2; and S4, preparing the anti-human CEACAM5 monoclonal antibody from the hybridoma cell line stably secreting the antibodies obtained in the step 3. The monoclonal antibody can be used to prepare a composition for treating a malignant tumor. The anti-human CEACAM5 monoclonal antibody can be widely used in various routine operations such as flow cytometry, immunohistochemical staining and immunoprecipitation.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Apparatus for and method of processing biological samples
ActiveUS20130040376A1Bioreactor/fermenter combinationsHeating or cooling apparatusWestern blotGenomic DNA
The present invention provides systems, devices, apparatuses and methods for automated bioprocessing. Examples of protocols and bioprocessing procedures suitable for the present invention include but are not limited to: immunoprecipitation, chromatin immunoprecipitation, recombinant protein isolation, nucleic acid separation and isolation, protein labeling, separation and isolation, cell separation and isolation, food safety analysis and automatic bead based separation. In some embodiments, the invention provides automated systems, automated devices, automated cartridges and automated methods of western blot processing. Other embodiments include automated systems, automated devices, automated cartridges and automated methods for separation, preparation and purification of nucleic acids, such as DNA or RNA or fragments thereof, including plasmid DNA, genomic DNA, bacterial DNA, viral DNA and any other DNA, and for automated systems, automated devices, automated cartridges and automated methods for processing, separation and purification of proteins, peptides and the like.
Owner:LIFE TECH CORP
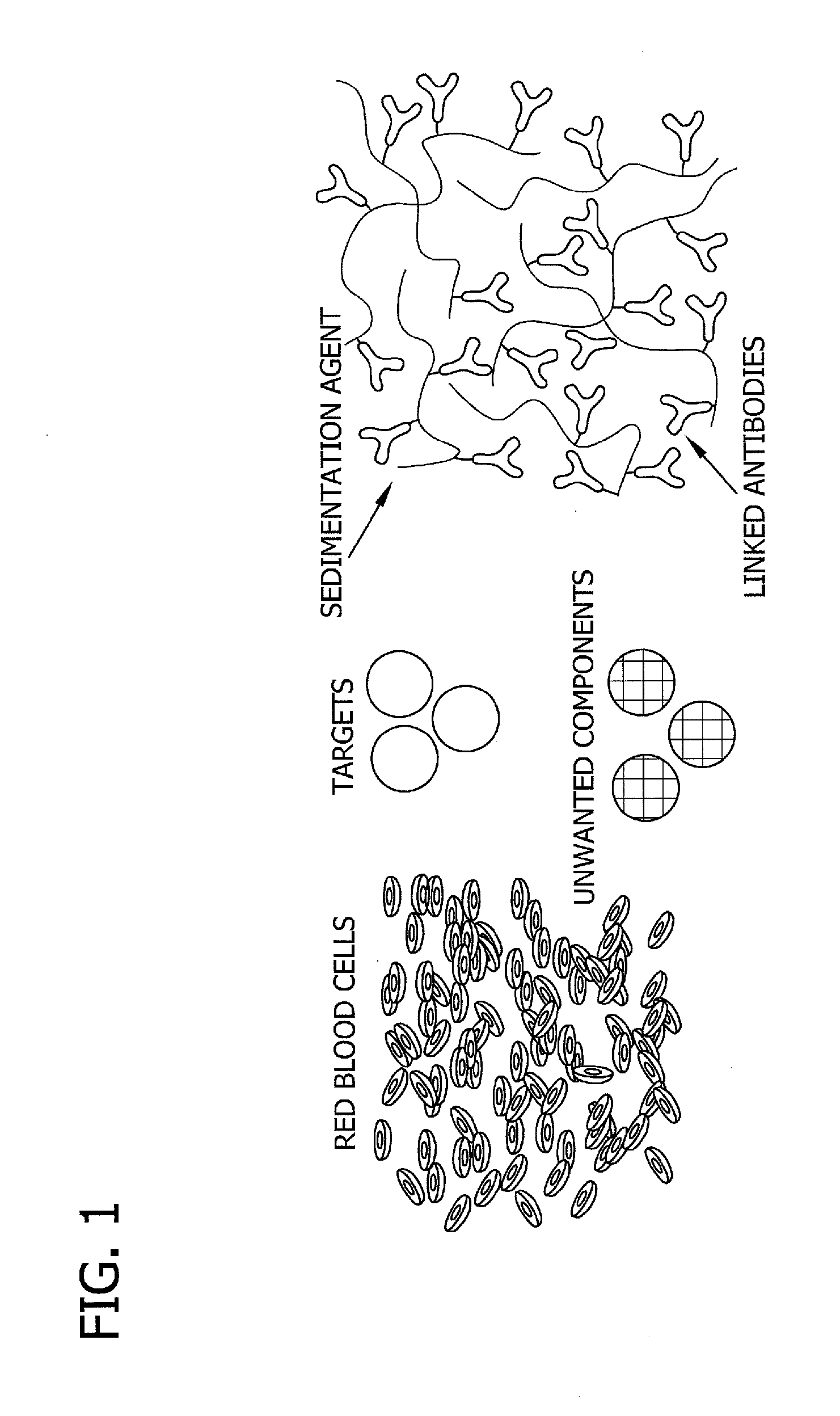
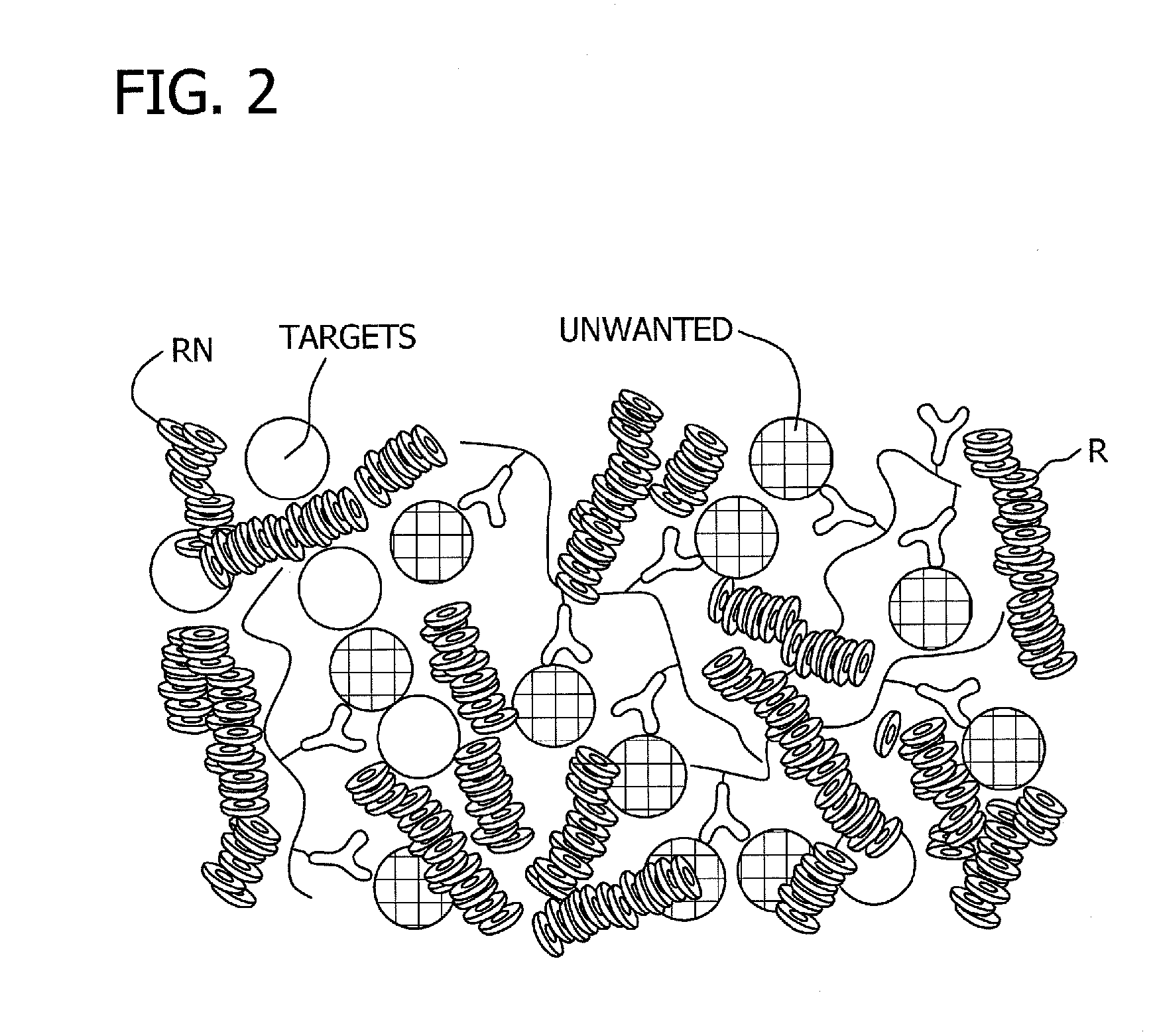
Antibody-linked immuno-sedimentation agent and method of isolating a target from a sample using same
InactiveUS20130266930A1Microbiological testing/measurementBiological material analysisRed blood cellAntigen binding
The present disclosure is directed to antibody-linked immuno-sedimentation agent, the antibody being linked to a sedimentation agent by a non-antigen binding region of the antibody, and a method of isolating a target from a sample using the antibody-linked immuno-sedimentation agent. The methods involve forming a mixture including a sample with an antibody linked immuno-sedimentation agent and red blood cells under conditions sufficient to form red blood cell rouleaux and allow antibody-antigen binding.
Owner:CYTOSED
Detection of Antibodies
InactiveUS20090029388A1Efficient methodImprove the signal-to-background ratioBiological testingAutoimmune diseaseWater Channel Proteins
The present invention relates to a method for detecting antibodies against a target antigen in a sample which comprises contacting the sample with labelled target antigen, subjecting the sample to immunoprecipitation to precipitate antibodies in the sample and detecting the presence of antibodies against the target antigen in the sample by means of the presence of labelled target antigen in the immunoprecipitate, wherein the labelled target antigen is a fusion protein comprising the target antigen and a fluorescent protein label and the presence of labelled target antigen in the immunoprecipitate is detected by means of the fluorescence of the fluorescent label. The method is particularly suitable for use where the target antigen is an autoantigen and can also be used to identify autoantigens implicated in a particular autoimmune disorder by screening serum samples from patients with a clinical phenotype indicative or suggestive of an autoimmune disorder and suitable controls. The target protein may be from the cys-loop acetyl choline receptor ion channel gene superfamily, the voltage-gated calcium, sodium or potassium ion channel gene superfamily, the glutamate receptor gene family, a receptor tyrosine kinase, or other membrane associated channels such as aquaporin gene family.
Owner:BEESON DAVID
Screening and application of plant antiviral new target PsbO1
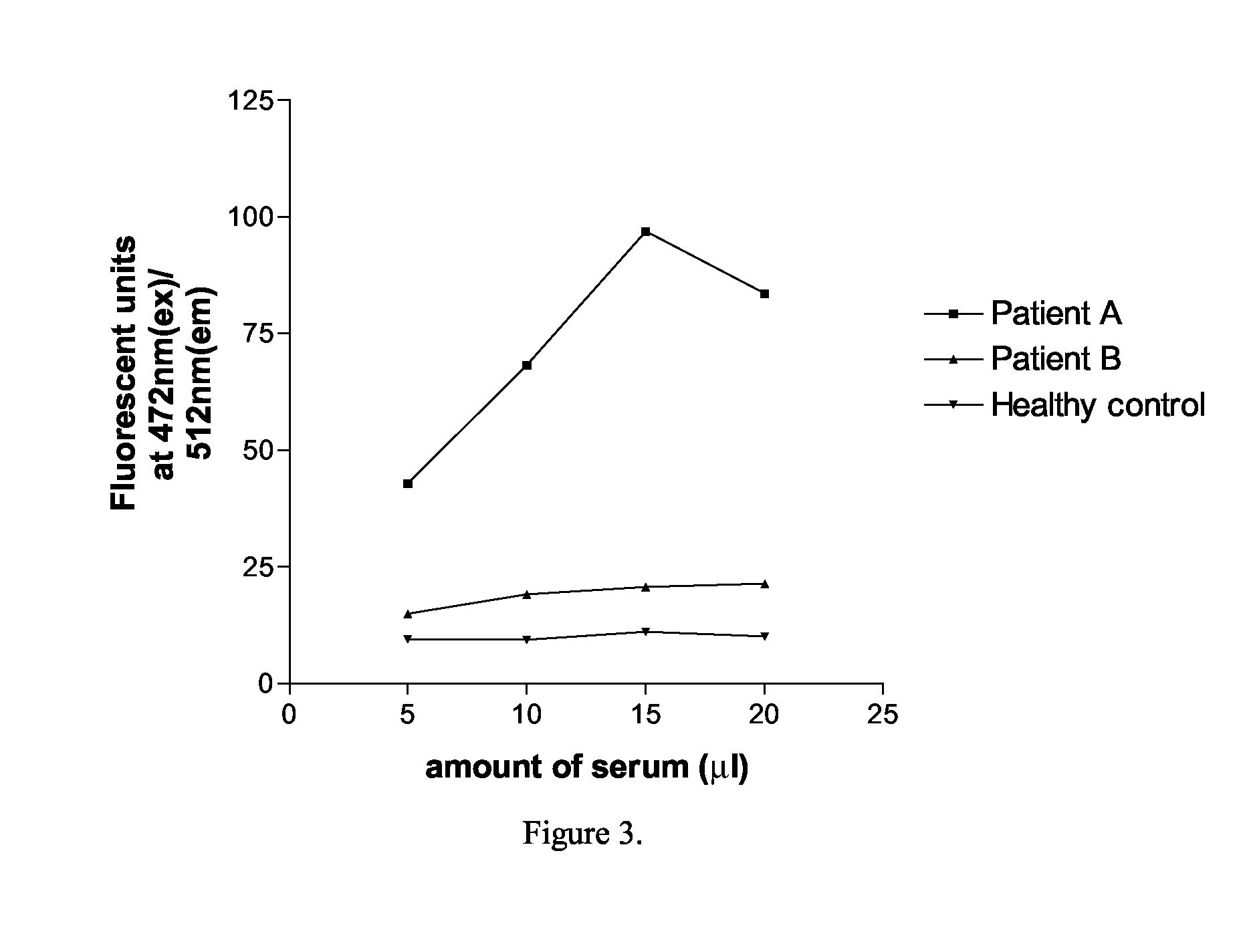
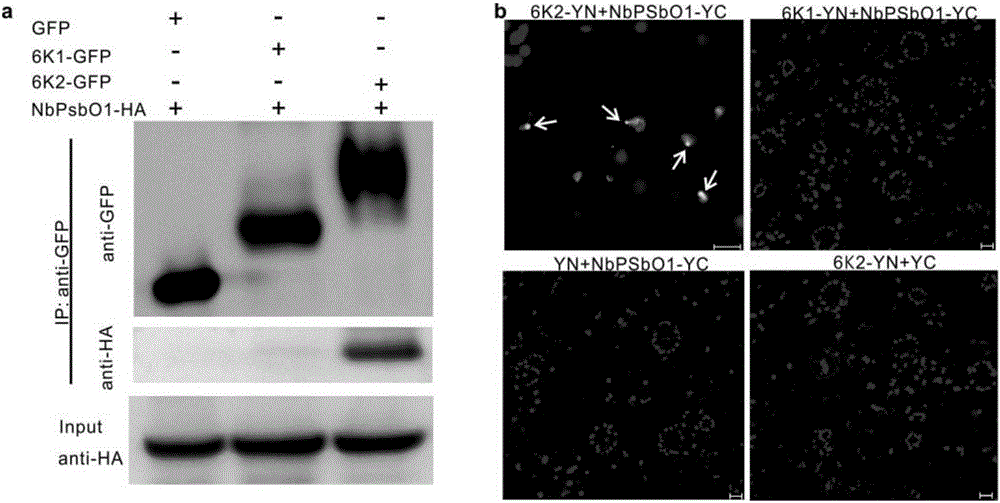
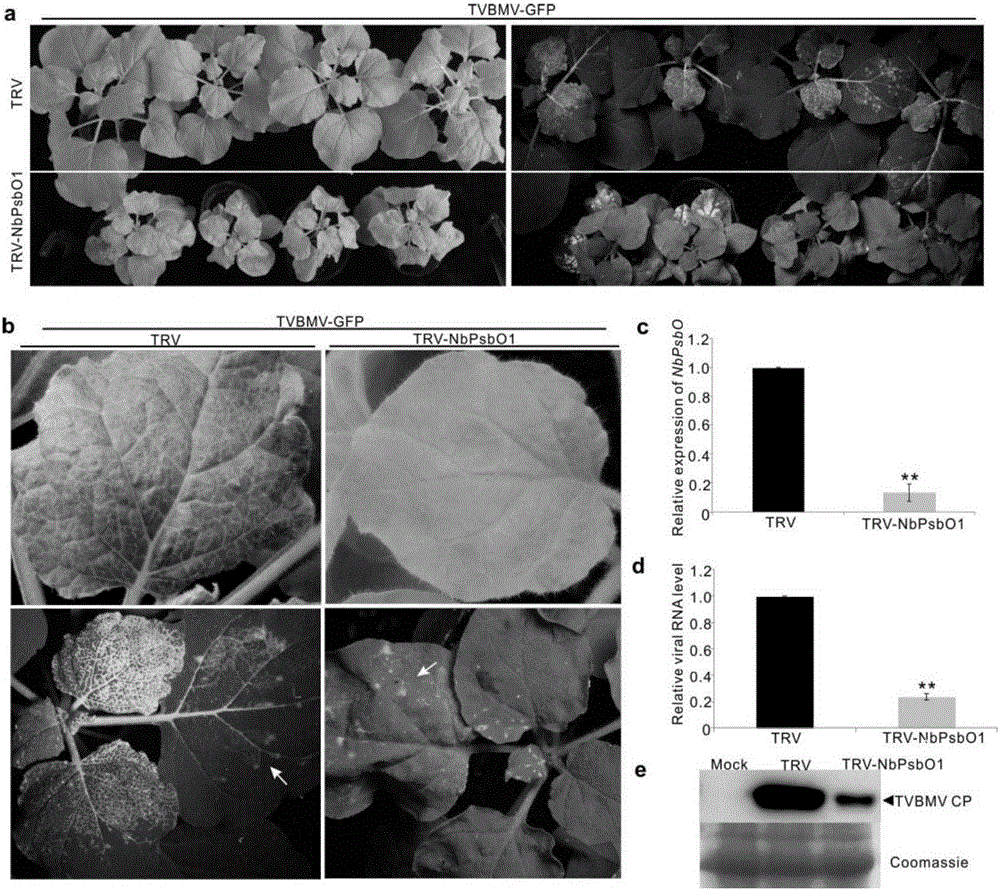
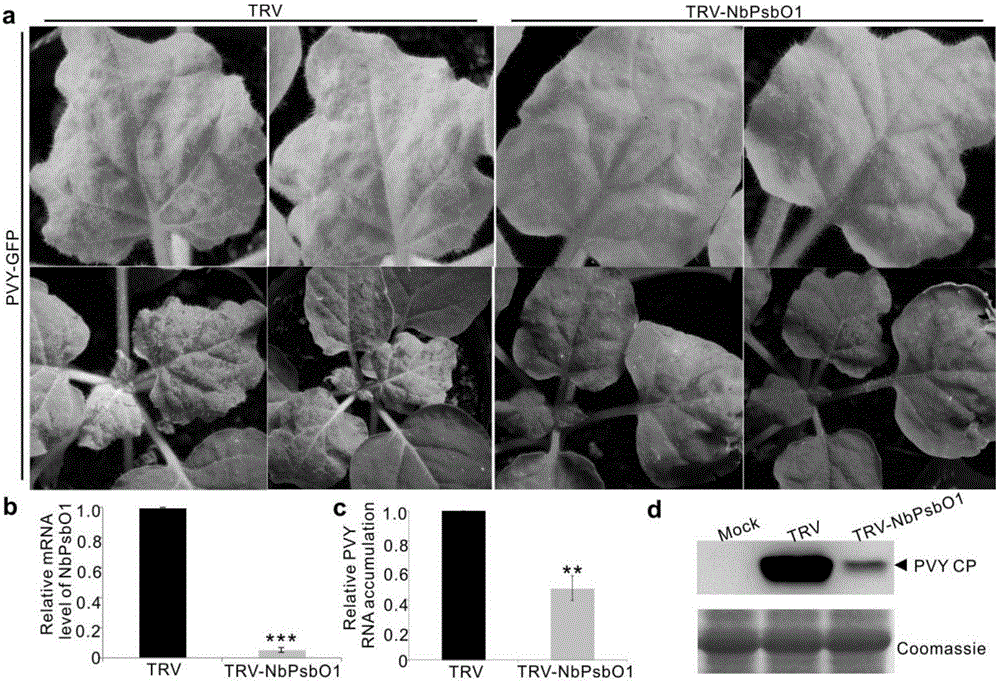
InactiveCN106699857AReduce infestationPlant peptidesGenetic engineeringNicotiana tabacumPotato virus Y PVY
The invention relates to the field of plant antiviral genetic engineering, and provides a plant antiviral new target. On the basis of tobacco vein banding mosaic virus infection cloning, photosystem II oxygen-evolving complex protein PsbO1 is screened through immunoprecipitation and mass spectrography, and a PbsO1 gene is obtained through reverse transcription PCR amplification. Immunoprecipitation and bimolecular fluorescence complementation prove that the PsbO1 can interact with the tobacco vein banding mosaic virus 6K2. Reduction in expression of the PsbO1 gene enables an infected plant to generate resistance to the tobacco vein banding mosaic virus and potato virus Y.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
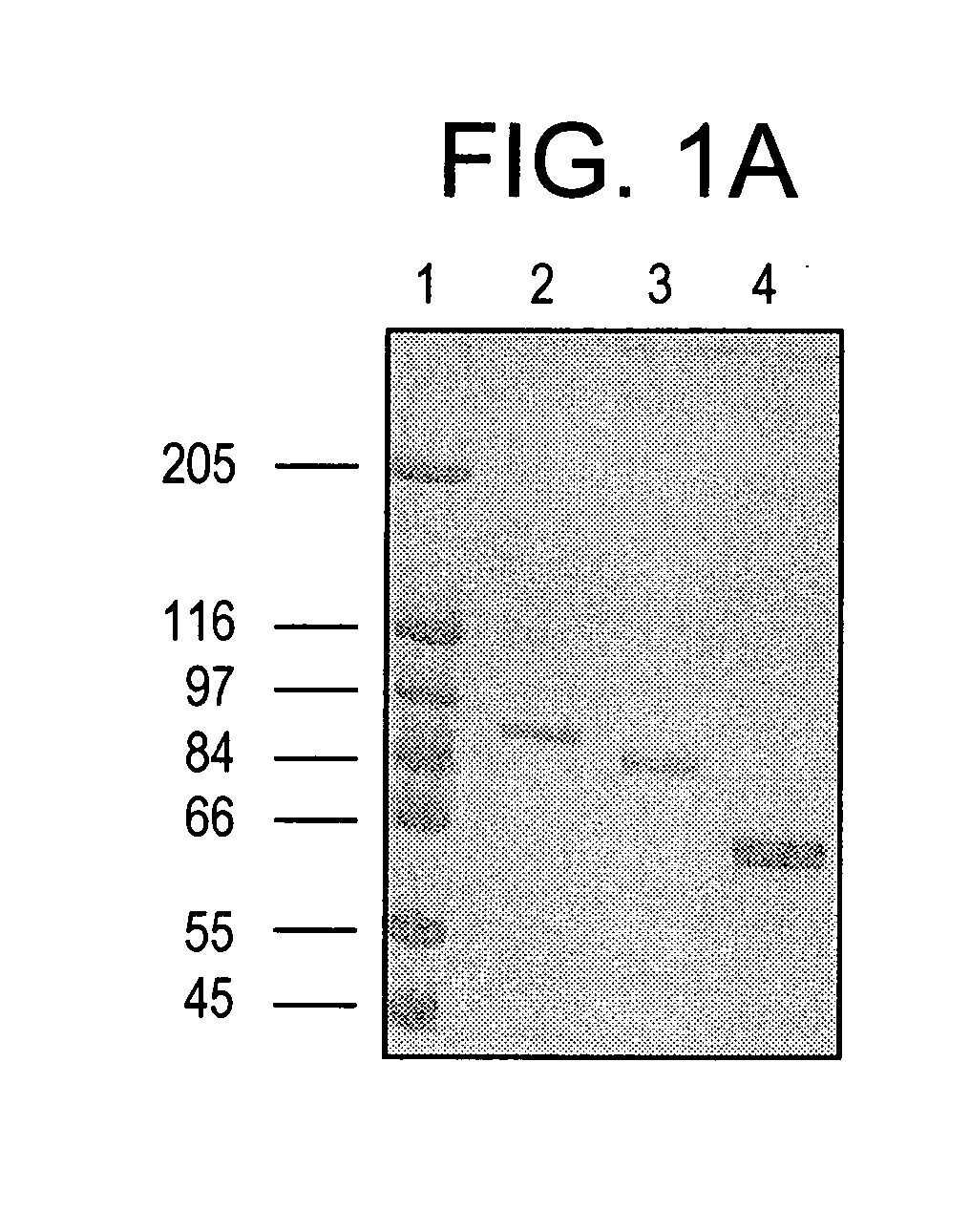
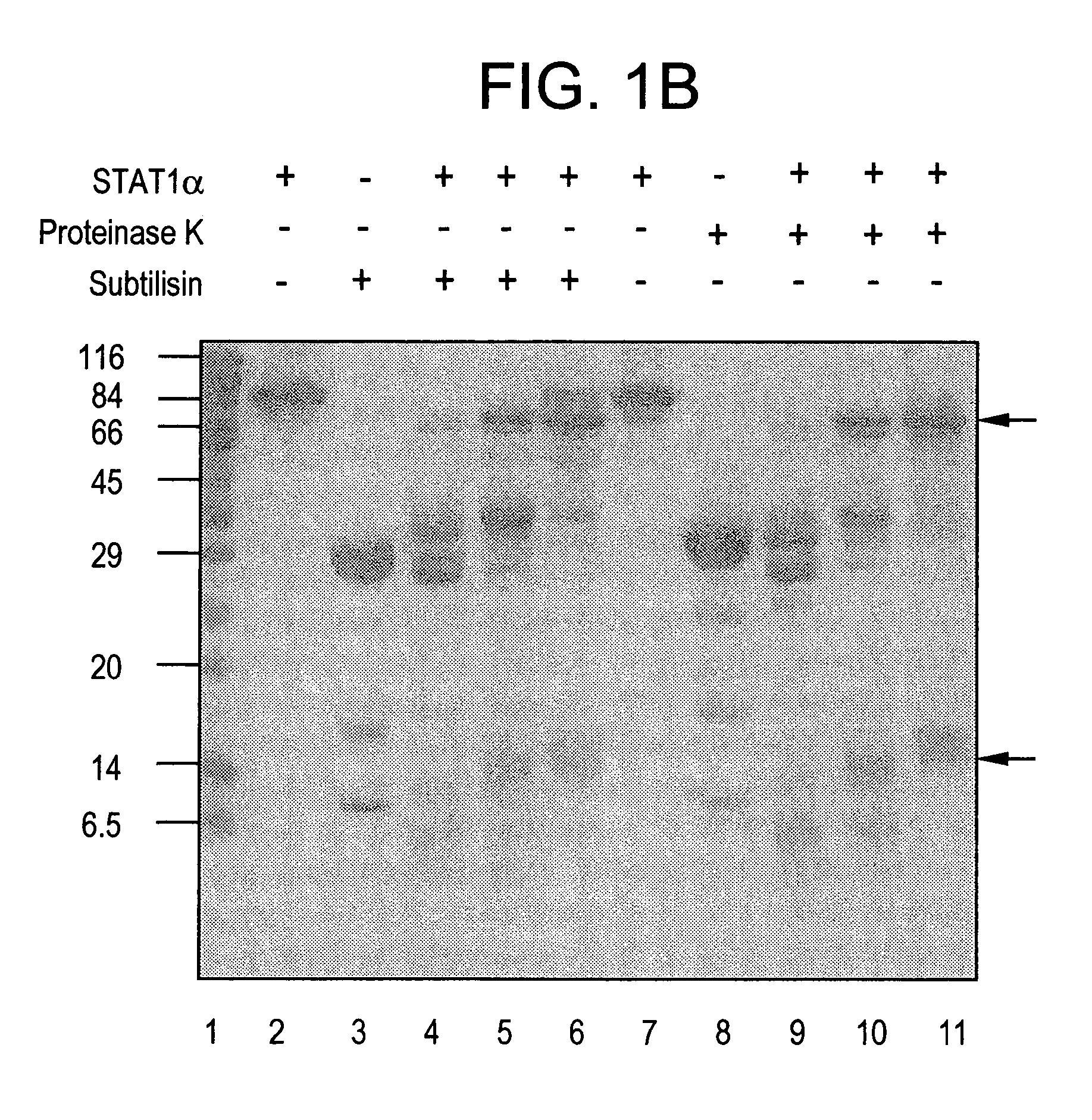
Purified Stat proteins and methods of purifying thereof
InactiveUS20060160152A1High binding affinityImprove throughputCompound screeningVirusesEscherichia coliCysteine thiolate
The present invention describes methods of producing milligram quantities of three forms of purified Stat1 protein from recombinant DNA constructs. In addition, the Stat proteins may be isolated in their phosphorylated or nonphosphorylated forms (Tyr 701). The proteins can be produced in baculovirus infected insect cells, or E. coli. A compact domain in the amino terminus of Stat1α was isolated and found to enhance DNA binding due to its ability to interact with a neighboring Stat protein. A relatively protease-resistant recombinant truncated form of the Stat protein was isolated in 40-50 mg quantities. Purification of the Stat proteins were performed after modifying specific cysteine residues of the Stat proteins to prevent aggregation. Activated EGF-receptor partially purified from membranes by immunoprecipitation was shown to be capable of in vitro catalysis of the phosphorylation of the tyrosine residue of Stat1 known to be phosphorylated in vivo. Techniques are enclosed to separate the phosphorylated from the nonphosphorylated Stat proteins. The techniques disclosed are general for Stat proteins and may be used to isolate large quantities of purified Stat 2, 3, 4, 5A, 5B and 6. Methods for using purified Stat proteins, truncated Stat proteins, or Stat N-terminal fragments for drug discovery are also disclosed.
Owner:THE ROCKEFELLER UNIV
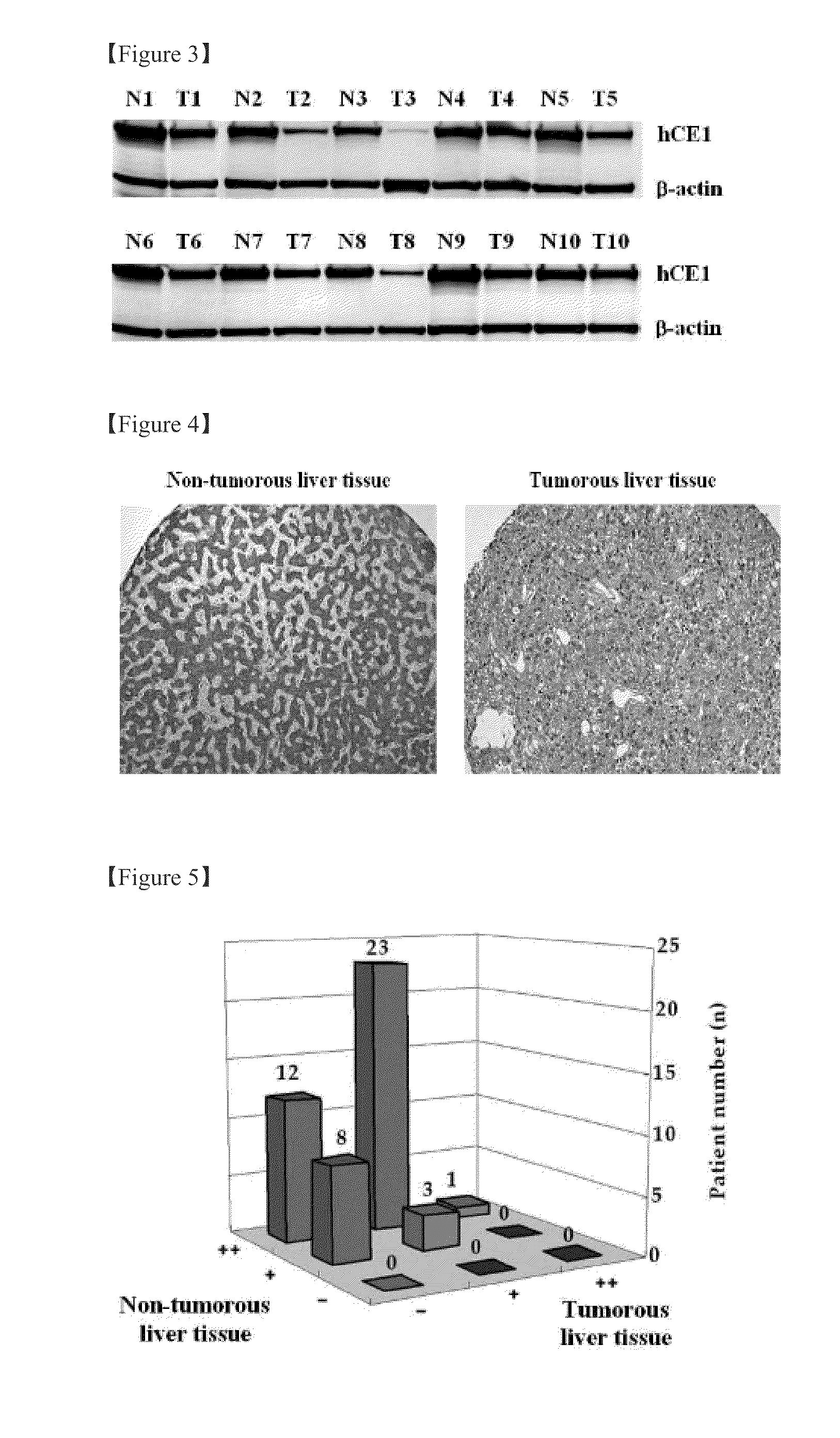
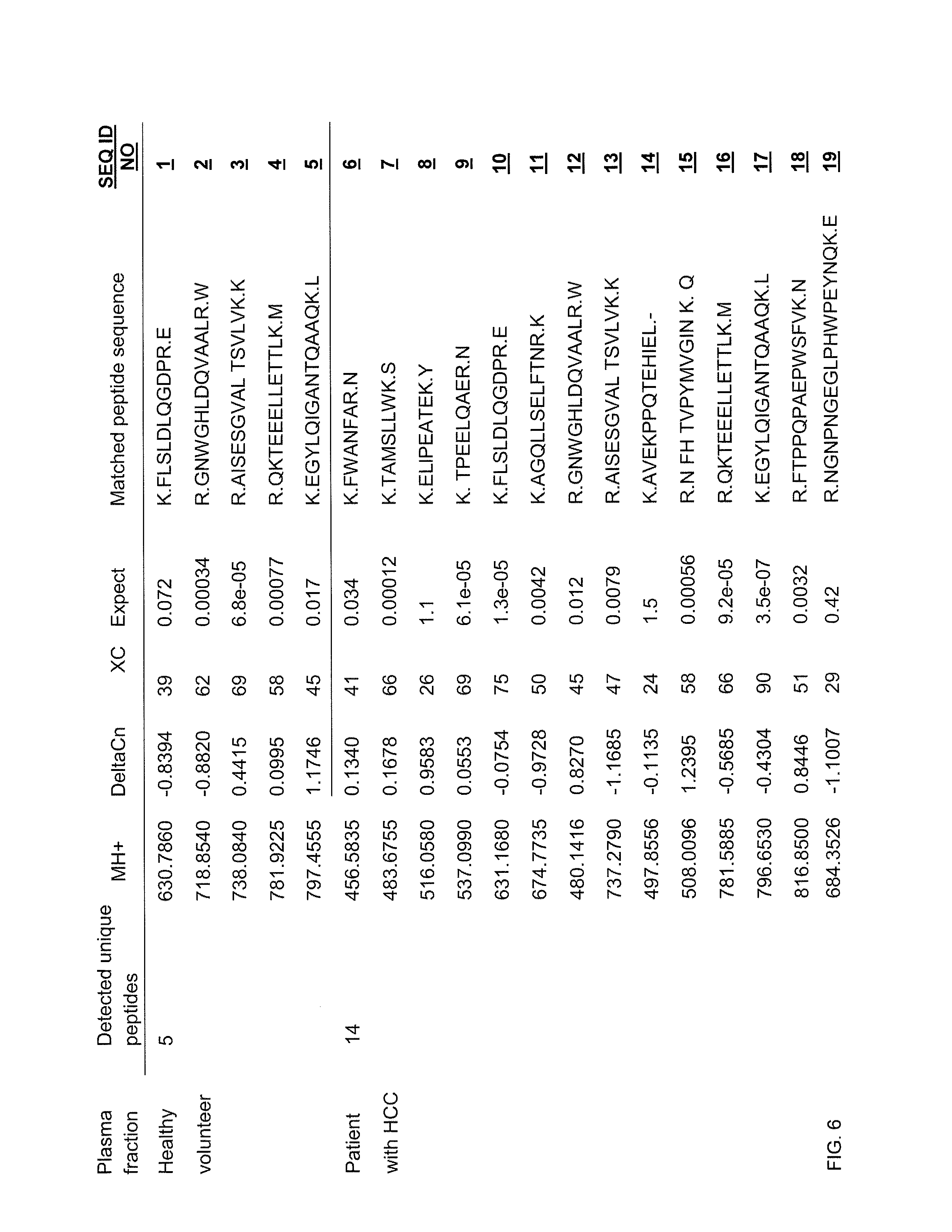
Plasma biomarker tool for the diagnosis of liver cancer comprising liver carboxylesterase 1 and liver cancer screening method
ActiveUS8198038B2Efficient detectionMicrobiological testing/measurementBiological material analysisFluorescenceScreening method
The present invention relates to a plasma biomarker for diagnosing hepatocellular carcinoma (HCC), in particular to the discovery of a protein in plasma using 2-D fluorescence differential gel electrophoresis (2-D DIGE), immunoprecipitation and Nano-liquid chromatography mass spectrometry (Nano-LC-MS / MS) system that was unknown on the basis of conventional techniques. By demonstrating the presence of liver carboxylesterase 1 (hCE1) in human plasma and confirming that its secretion level is higher in patients with HCC than in healthy volunteers, this invention may be used as a screening method to diagnose HCC at an early stage.
Owner:IND ACADEMIC COOP FOUND YONSEI UNIV
Method of identifying active chromatin domains
The invention provides a method of mapping DNA-protein interactions within a genome by fixing living cells to cross-link DNA and proteins, lysing the cells, and isolating chromatin by immunoprecipitation. DNA is purified and a SAGE protocol is performed on the purified DNA to produce GMAT-tag sequences, which are compared to a genomic sequence of the living cells to map DNA-protein interactions. The invention further provides a method of identifying an active chromatin domain and a method of identifying aberrant chromatin acetylation, wherein chromatin immunoprecipitation is performed using an antibody recognizing acetylated histone protein.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Anti-human phylaxin synthetic polypeptide antibody and its preparation method
InactiveCN1594363AHigh potencyHigh affinityImmunoglobulins against animals/humansResistinBiologic Products
The invention discloses an anti-human phylaxin synthetic polypeptide antibody and its preparation method which consists of, synthesizing polypeptides using one segment of the resistin amino acid sequence, using its amino acid sequence CSMEEAINERIQE as semiantigen, preparing artificial immunity antigen through connection with protein carriers, immunizing the animals so as to effectively induce organism immune response, thus obtaining the antibodies.
Owner:INST OF RADIATION MEDICINE CHINESE ACADEMY OF MEDICAL SCI
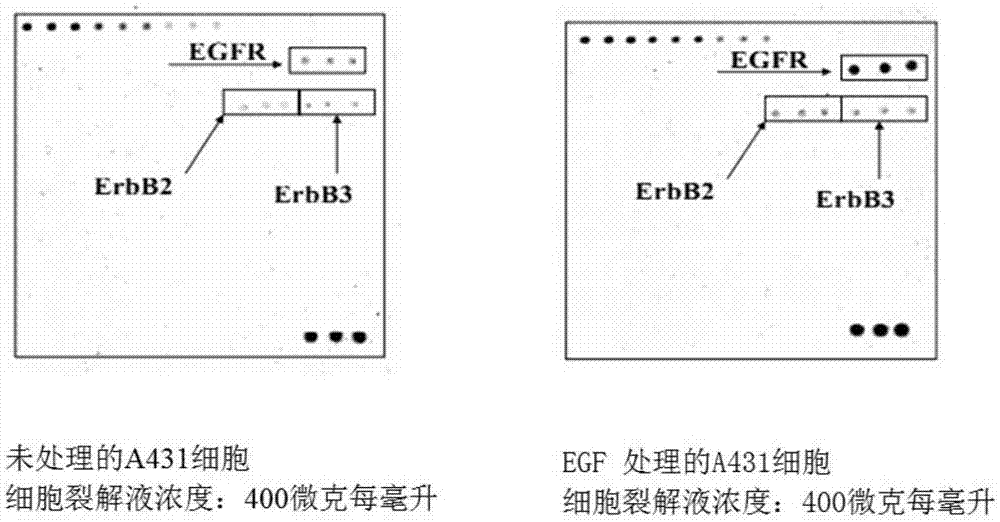
Phosphorylation antibody chip kit for detecting human receptor tyrosine kinase (RTK)
ActiveCN106918698ASimplified steps for fixing to glass slidesHigh sensitivityBiological material analysisBiological testingBiotin-streptavidin complexPhosphorylation
The invention relates to a phosphorylation antibody chip kit for detecting human RTK. The phosphorylation antibody chip kit for detecting human RTK comprises: a solid-phase vector, which is a standard film or slide coated with a specific antibody; a cleaning solution, which comprises a 20X concentrated cleaning solution of 0.1% Tween-20 and a dilution solution thereof; a sample diluting solution; a dilution solution used for diluting the antibody and HRP-streptavidin; a biotinylated detection antibody mixture; a 300X concentrated fluorescein-streptavidin solution; a sample treating solution, which is a 2X cell lysis buffer; and the specific antibody, which is targeted to 71 proteins. The RTK phosphorylation antibody chip kit provided by the invention can rapidly, simply and accurately determine the situations of phosphorylation of 71 proteins in a RTK pathway through one experiment; and pathway activation in cells can be rapidly detected by monitoring the phosphorylation changes of proteins in an experimental model system without complicated immunoprecipitation and blotting.
Owner:RAYBIOTECH INC GUANGZHOU
Lyophilized viper antivenin and preparation method thereof
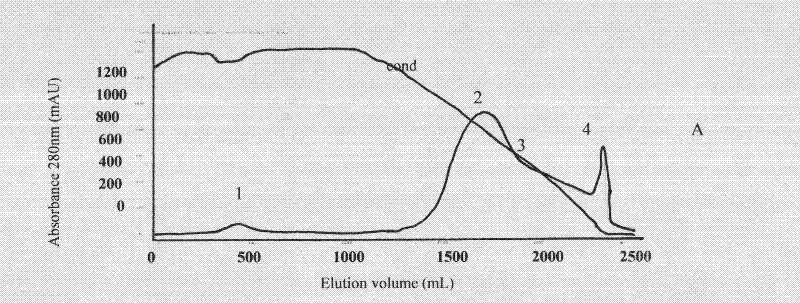
ActiveCN101816789BHigh potencyGuaranteed curative effectAntinoxious agentsMammal material medical ingredientsBlood plasmaImmunoprecipitation
The invention discloses a lyophilized viper antivenin and a preparation method thereof, belonging to biochemical products, more particularly relating to an antivenin and a preparation technology thereof. The mass ratio of the antivenin and viper venom is 15:1, which can specifically neutralize the viper venom injected to mouse; as determined by immunodiffusion, the immunoprecipitation line appears in case that the ratio of viper antivenin F(ab')2 in lyophilized form to viper venom is 8:1; and other detected items conform to the quality standard of antivenin in Chinese Pharmacopoeia 2010. The detection of Phenyl-Sepharose (low-sub) FF column chromatography result shows that: activity is centralized at eluting peak 1, micromolecule impurity proteins are centralized at penetration peal, eluting peak 2 and eluting peak 3. According to the technology of the invention, immune blood plasma is resulted from viper venom immune horse, IgG is prepared by salting out the immune blood plasma, and F(ab')2 active fragment is obtained after the IgG is subject to enzymolysis and purification by a hydrophobic column. The lyophilized viper antivenin has strong specificity, high potency and more than85% of the purity of antivenin F(ab')2 in lyophilized form.
Owner:浙江健博生物科技股份有限公司
Few-sample whole genome DNA (deoxyribonucleic acid) methylation detection method and kit
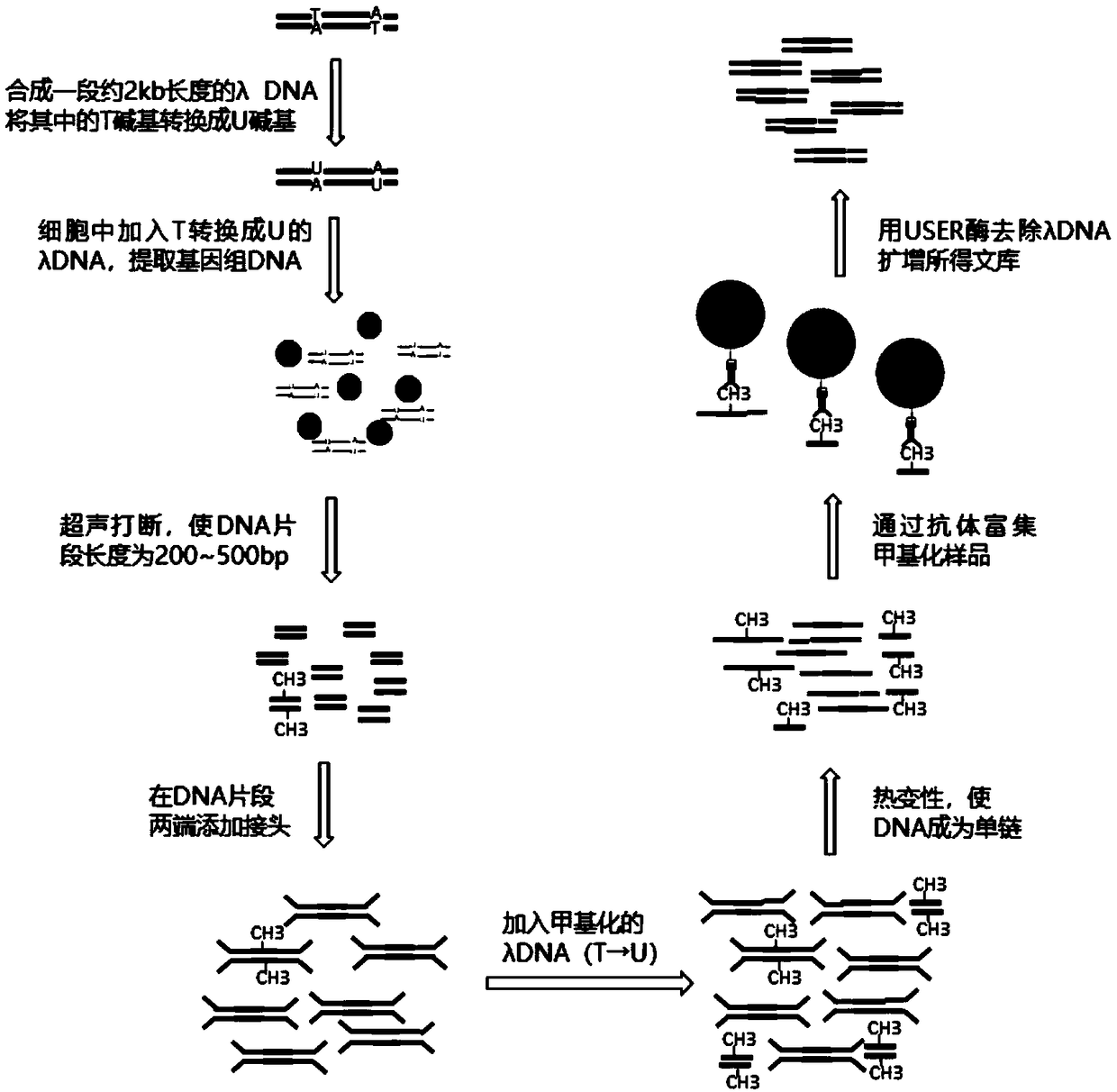
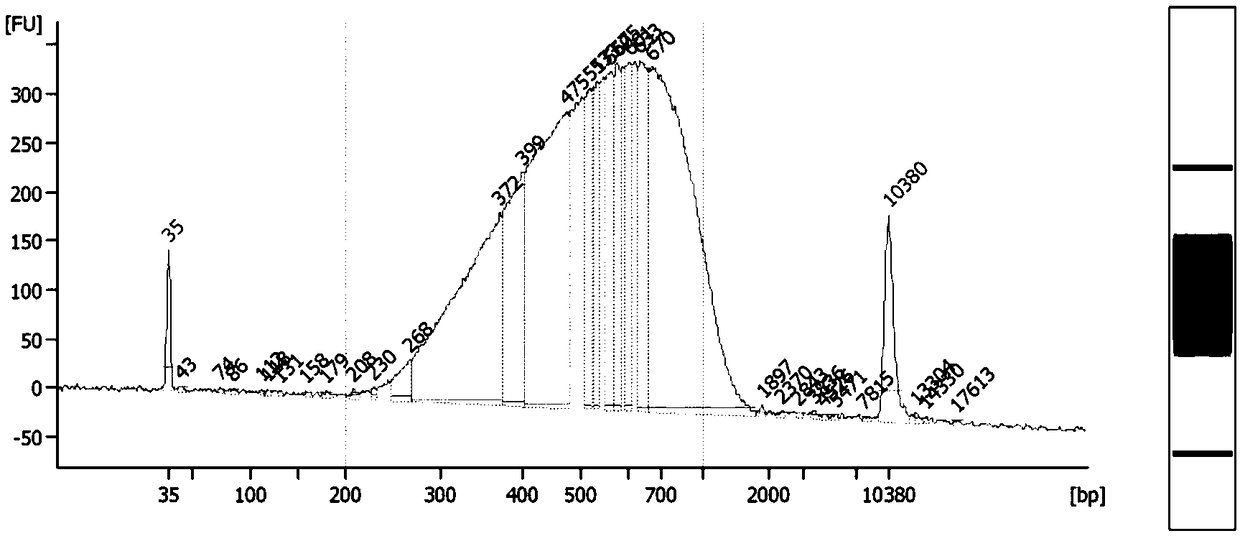
InactiveCN108796057AThe operation method is simple and efficientLow costMicrobiological testing/measurementDNA methylationImmunoprecipitation
The invention discloses a few-sample whole genome DNA (deoxyribonucleic acid) methylation detection method, and relates to a method for detecting less-sample (300pg DNA or 50 cells) whole genome DNA methylation by an improved MeDIP-Seq technique based on high-throughput sequencing. According to the method, lambda DNA containing dUTP is added into a small number of initial samples to reduce sampleloss, base-building and immuno-precipitation efficiency is improved, MeDIP-DNA is digested by USER enzyme, so that added methylation lambda DNA is removed, and a methylation sample sequencing libraryto be detected cannot be polluted by the methylation lambda DNA. According to the method, needed sample amount is 300pg DNA or 50 cells, the method is more applicable to MeDIP-Seq analysis of a smallnumber of source samples, operation method of a MeDIP experimental technique and a kit matched with the experimental technique is simple and convenient, specific commercial kits and experimental devices are omitted, cost is low, and the method has wide applicability and universality.
Owner:SHANGHAI JIAO TONG UNIV
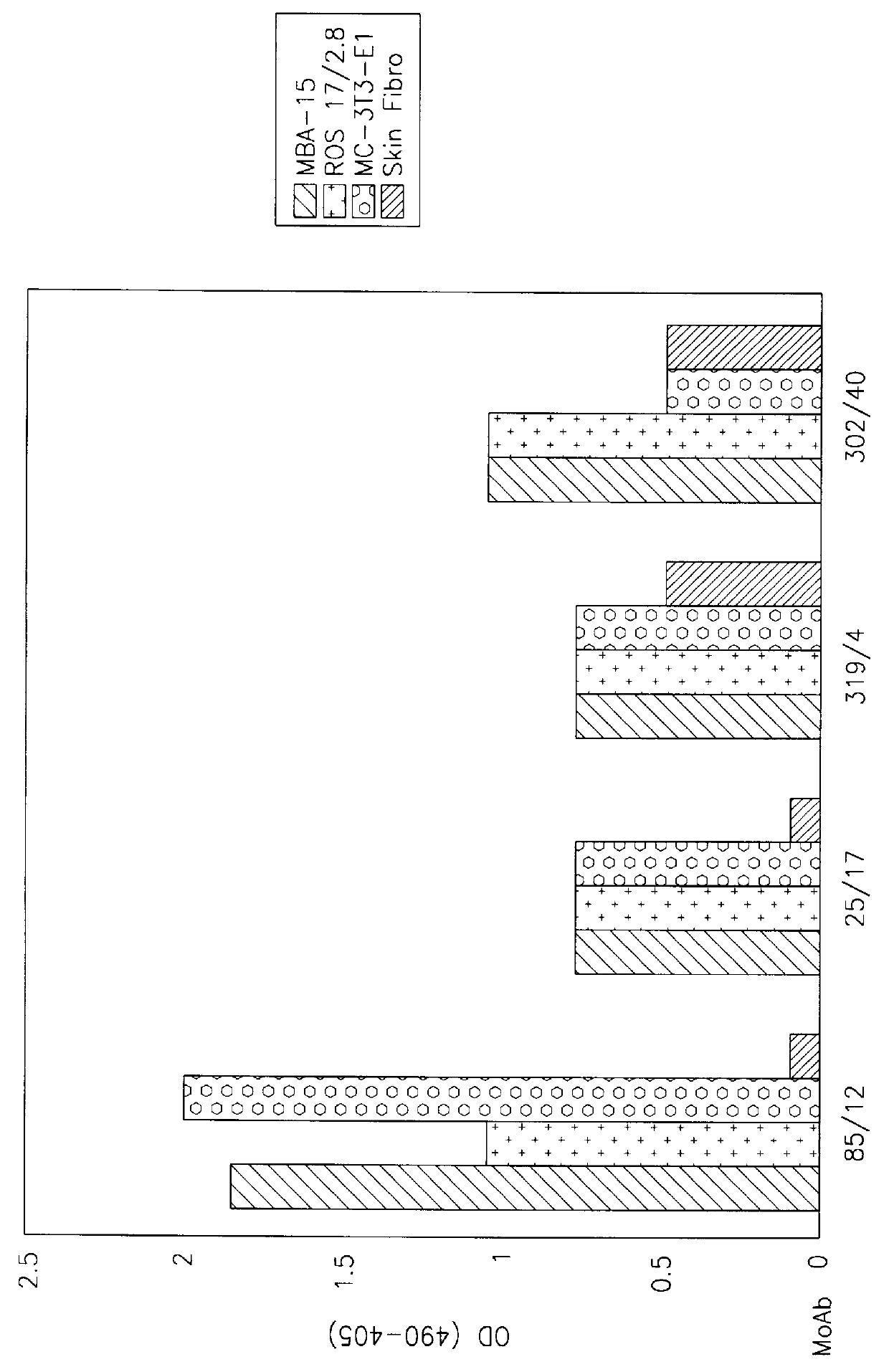
Osteoblast and fibroblast antigen and antibodies recognizing it
Monoclonal antibodies (MAb) are provided which have binding specificity to an antigen expressed on osteogenic and fibroblastic cells (OFA) and are capable of binding to osteogenic and fibroblastic cells at a substantially higher extent as compared to their binding to skin fibroblasts and stromal adipocytes. Also provided is a novel osteogenic and fibroblastic antigen (OFA) which is expressed on osteogenic and fibroblastic cells at a higher level than its expression in skin fibroblasts and stromal adipocytes having a molecular weight of about 80 kD as determined by western blotting or immunoprecipitation. The OFA and anti-OFA-MAbs are useful in the diagnosis and treatment of various hone related conditions.
Owner:RAMOT UNIV AUTHORITY FOR APPLIED RES & INDAL DEVMENT
Method for gathering phosphorylated proteins and method for detecting phosphorylated proteins
InactiveCN109916701AAdsorption and elution conditions are mildAvoid sex changePreparing sample for investigationMaterial analysis by electric/magnetic meansCrystallographyEnrichment methods
The invention provides a method for gathering phosphorylated proteins and method for detecting phosphorylated proteins and belongs to the technical field of phosphorylated protein detection. The method comprises steps that a phosphorylated protein sample and the buffer solution are mixed with a chitosan functionalized magnetic material, after magnetic separation, a phosphorylated protein-enrichedmagnetic material is obtained; the phosphorylated protein-enriched magnetic material is mixed with the acid solution, after magnetic separation, the phosphorylated protein-enriched solution is obtained; a core of the chitosan functionalized magnetic material is Fe3O4, and an outer layer of Fe3O4 is sequentially wrapped by silica and chitosan polymeric materials. The method is advantaged in that the method can overcome limitations of a traditional immunoprecipitation enrichment method for antibody and has wide applicability.
Owner:INST OF ENVIRONMENTAL MEDICINE & OCCUPATIONAL MEDICINE ACAD OF MILITARY MEDICINE ACAD OF MILITARY SCI
Identification method of deer blood active crystal preparation
InactiveCN103421882AComplete and scientifically reliable identification methodMicrobiological testing/measurementMaterial analysis by electric/magnetic meansBlood componentImmunoprecipitation
The invention discloses an identification method of a deer blood active crystal preparation. The method comprises a PCR amplification test identification of specific DNA sequence of deer blood in the deer blood active crystal preparation, if a 405 bp stripe is amplified out, indicating that the preparation contains components of sika deer blood and red deer blood, if a 317 bp stripe is amplified out, indicating that the preparation contains components of sika deer blood; an immunoprecipitation test identification, if an immunoprecipitation line appears between each non-deer blood antibody immune serum and the deer blood active crystal preparation, indicating that the tested deer blood active crystal preparation contains other non-deer blood components; and an SDS-PAGE test identification for specific protein components contained in the deer blood active crystal preparation, if the processed gel appears feature spectrum bands, indicating that the deer blood active crystal preparation contains deer blood specific protein components. The method mentioned above provides a complete and scientific identification method for distinguishing true or false and high-quality or low-quality of deer blood crystal products in present markets.
Owner:苏州红冠庄国药股份有限公司
Peroxiredoxin 1 binding protein and application thereof
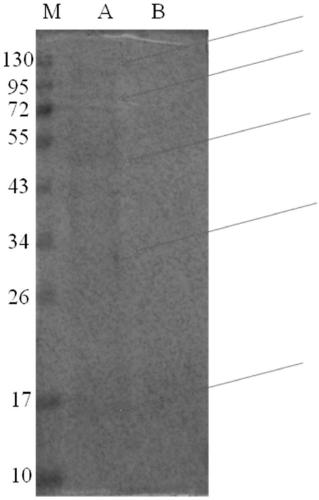
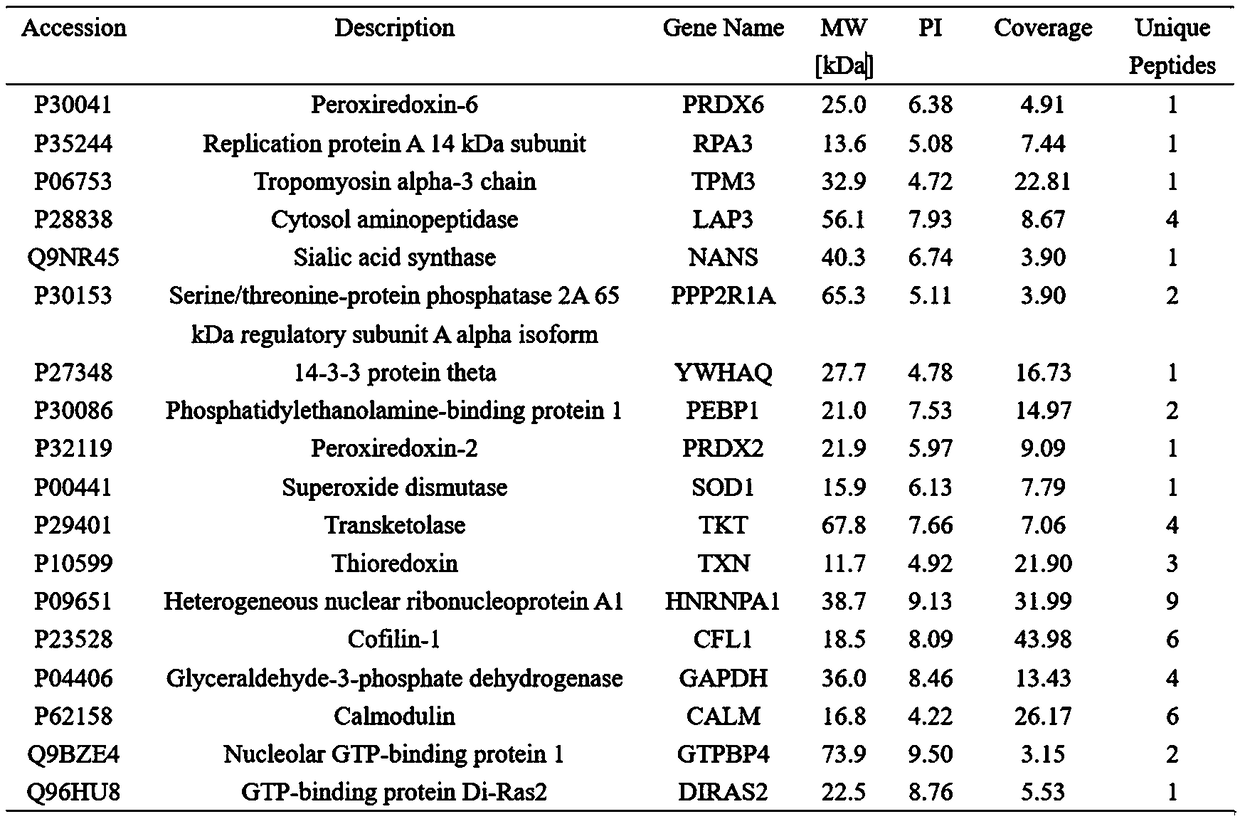
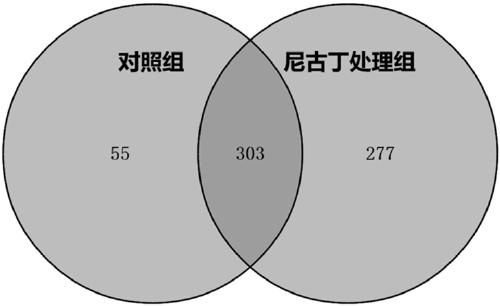
ActiveCN108949705APrevention and treatment of lesionsPrevention and treatment of tumorsHydrolasesMicrobiological testing/measurementImmunoprecipitationPrecancerous lesion
The invention discloses a peroxiredoxin 1 binding protein and application thereof, and the peroxiredoxin 1 binding protein comprises one or more of TXN (Trx), ASK1, TPM3, CFL1, GTPBP4, DIRAS2 and PPP2R1A. By immunoprecipitation assay, the peroxiredoxin 1 binding protein can be bonded to peroxiredoxin 1, and nicotine can promote the binding of the peroxiredoxin 1 and the peroxiredoxin 1 binding protein. In oral precancerous lesions such as leukoplakia cells, the expression of the peroxiredoxin 1 binding protein is significantly increased, and the nicotine can regulate the expression of the theperoxiredoxin 1 binding protein. The peroxiredoxin 1 binding protein can be used as a molecular marker for diagnosing or preventing the oral precancerous lesions such as oral leukoplakia, preparing aPrx1 protein conjugate, or binding, labeling, identifying, enriching, sorting or purifying Prx1 overexpresses cells.
Owner:BEIJING STOMATOLOGY HOSPITAL CAPITAL MEDICAL UNIV
Method for screening and identifying tumor/testis antigens based on monoclonal antibodies with spermatogenic cell specificities
InactiveCN102507937ASolve difficult-to-screen problemsComponent separationAntigenMonoclonal antibody 14G2A
The invention discloses a method for screening and identifying tumor / testis antigens based on monoclonal antibodies with spermatogenic cell specificities. Spermatogenic cells of human bodies are used as immune antigens, monoclonal antibodies are prepared by the aid of the lymphocyte hybridoma technology, the monoclonal antibodies with spermatogenic cell specificities are screened by the aid of immunohistochemistry, and then the expression distribution law of antigens identified on the screened monoclonal antibodies on tumor tissues and other normal tissues is researched on a protein level. The monoclonal antibodies are selected for the expression law of the tumor / testis antigens by the aid of immunohistochemical staining on the basis to be used as candidate antibodies for identifying the tumor / testis antigens, and new tumor / testis antigen molecules recognized by the monoclonal antibodies can be identified by the aid of proteomics technologies such as immunoprecipitation-mass spectrum analysis and the like.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
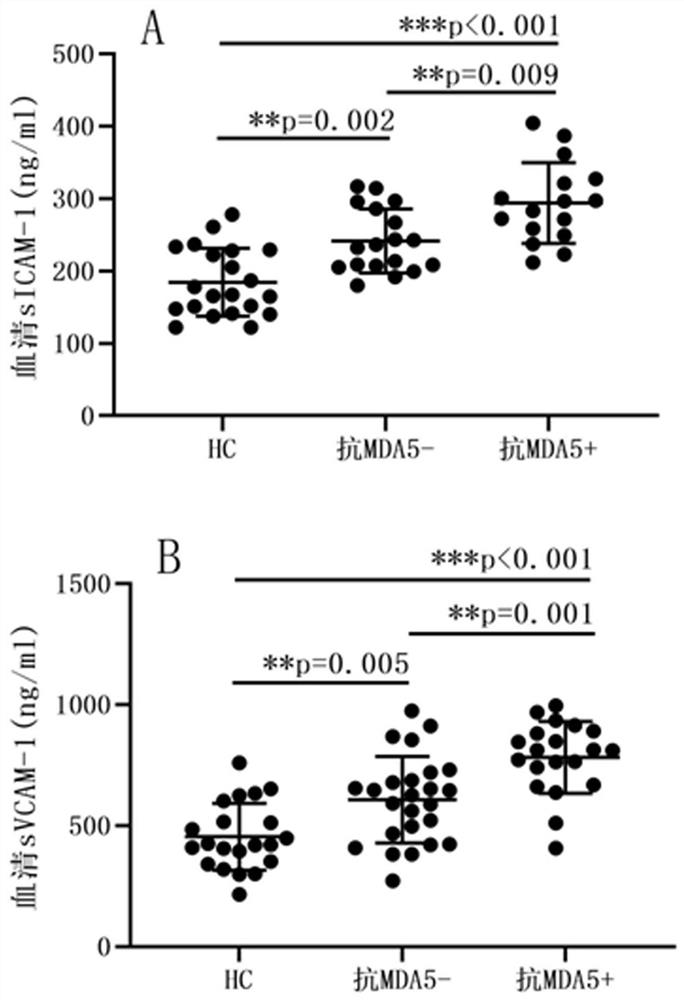
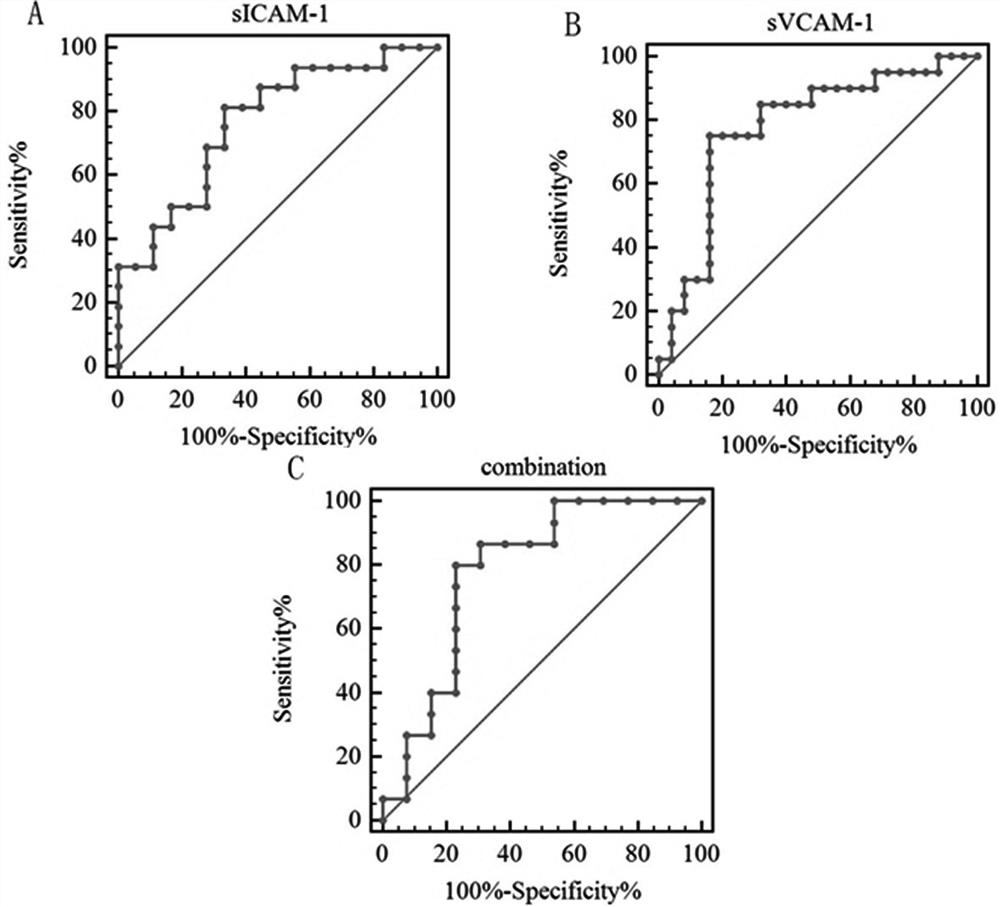
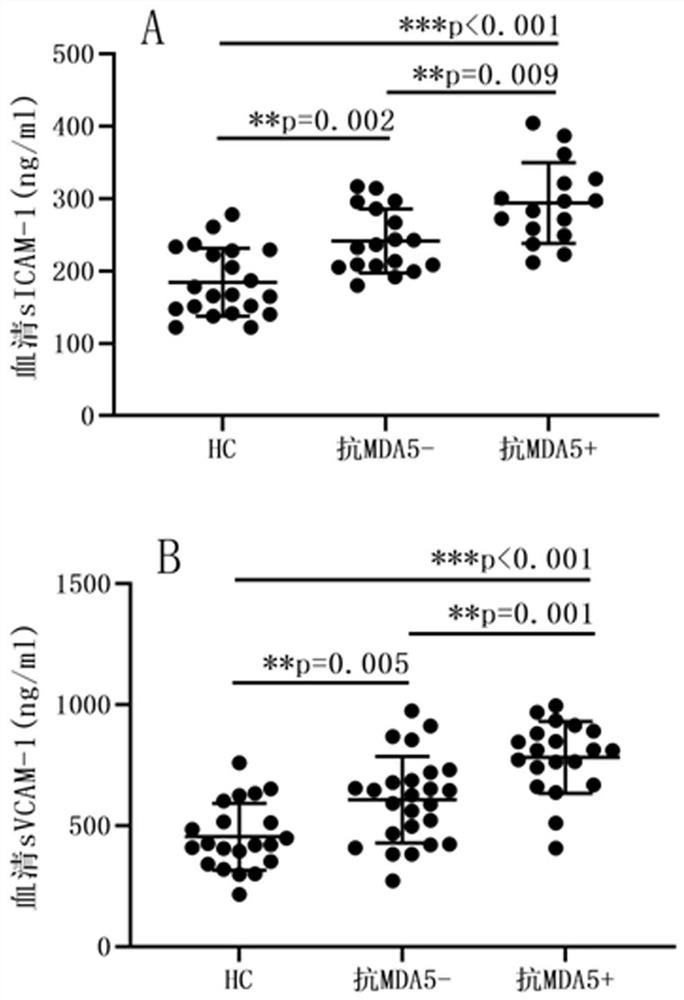
Molecular marker for diagnosing anti-MDA5 positive dermatomyositis and application thereof
PendingCN113960315ASolve the complex detection methodImprove diagnostic accuracyDisease diagnosisBiological testingDiseaseAntiendomysial antibodies
The invention belongs to the technical field of biological medicines, and particularly relates to a molecular marker for diagnosing anti-MDA5 positive dermatomyositis and application thereof. The invention relates to an application of a molecular marker in preparation of a diagnostic kit for diagnosing anti-MDA5 positive dermatomyositis. The molecular marker is a serum soluble intercellular adhesion molecule-1 (ICAM-1) and a vascular cell adhesion molecule-1 (VCAM-1). Experiments prove that ICAM-1 and VCAM-1 levels of dermatomyositis patients are higher than those of normal people, the levels of anti-MDA5 antibody positive dermatomyositis serum of the ICAM-1 and VCAM-1 are higher than those of anti-MDA5 antibody negative groups, ICAM-1 and VCAM-1 serve as markers for diagnosing anti-MDA5 positive dermatomyositis, and the problems that in the prior art, an anti-MDA5 antibody detection method is complex, kit manufacturing and standard substance selection are difficult, an immunoprecipitation method is complex in operation, and the detection method cannot be popularized in clinical application. The early detection of the increased adhesion molecule level is beneficial to diagnosis of anti-MDA5 antibody positive dermatomyositis, guidance of next treatment is facilitated, and the risk of disease deterioration and death of dermatomyositis patients is reduced.
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV
Immunomagnetic microparticle based on yelk antibody as well as preparation method and application of immunomagnetic nanoparticle
ActiveCN102086225AImprove separation efficiencyLarge specific surface areaSugar derivativesPeptide preparation methodsNanoparticleSuperparamagnetism
The invention relates to a preparation method and application of an immunomagnetic microparticle based on a yelk antibody (IgY). The yelk antibody (IgY) refers to an antibody separated from yelks of poultries such as chickens, ducks and gooses, and the magnetic microparticle refers to a magnetic microparticle with the particle diameter of 1 nm-200 mu m, superparamagnetism and high specific surface area. The application of the immunomagnetic microparticle comprises separation of large biological molecules such as protein, nucleic acid and the like in a biological sample, separation of cells and immunoprecipitation tests. In the preparation method, the yelk antibody (IgY) is immobilized on the surface of the magnetic microparticle so as to prepare the immunomagnetic microparticle based on the yelk antibody (IgY). The purpose of separating large biological molecules and cells from the biological sample is achieved by using affinity of large biological molecules and corresponding yelk antibodies thereof as well as superparamagnetism of immunomagnetic microparticles.
Owner:XIAN GOLDMAG NANOBIOTECH
Multiplex immuno screening assay
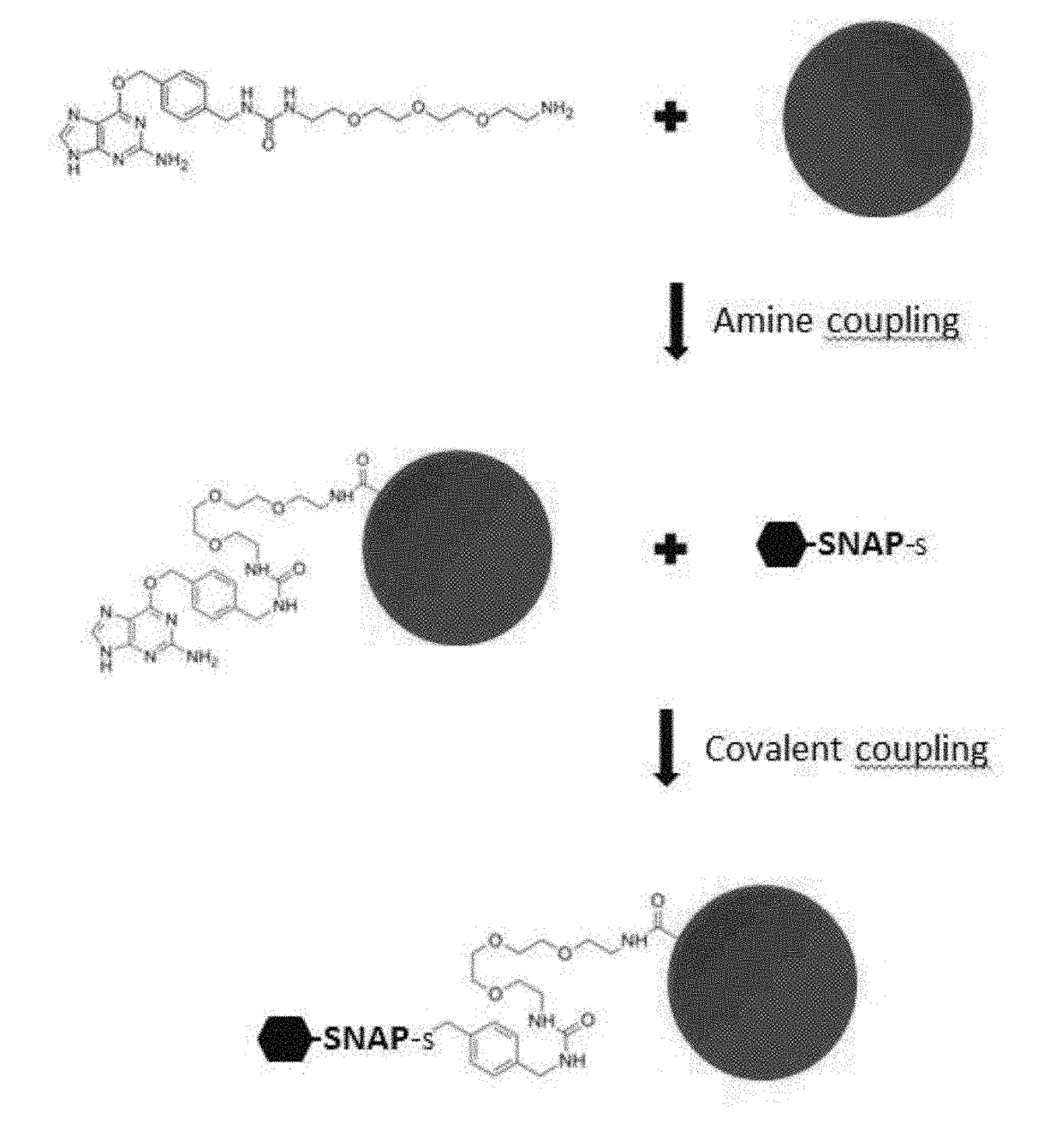
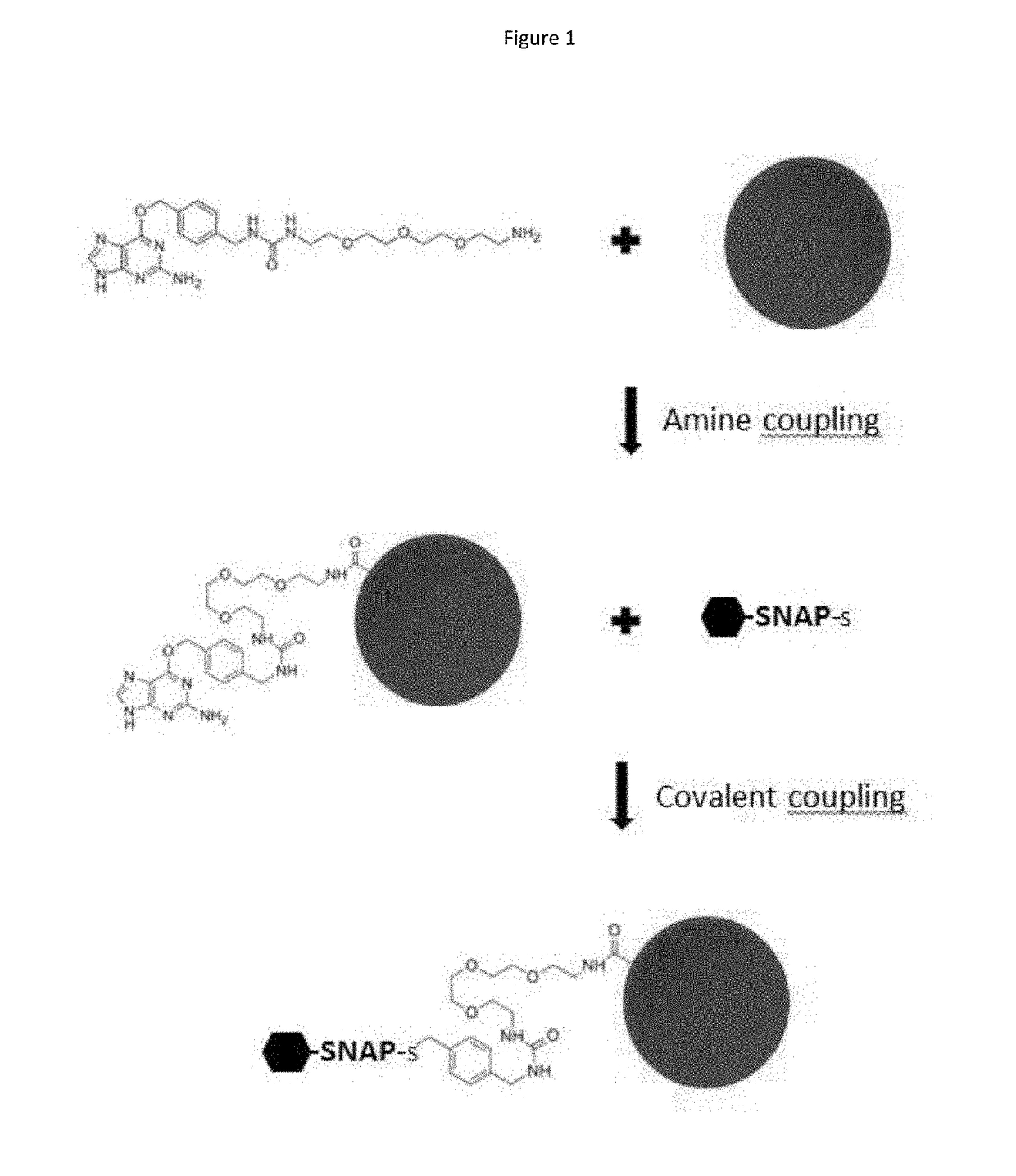
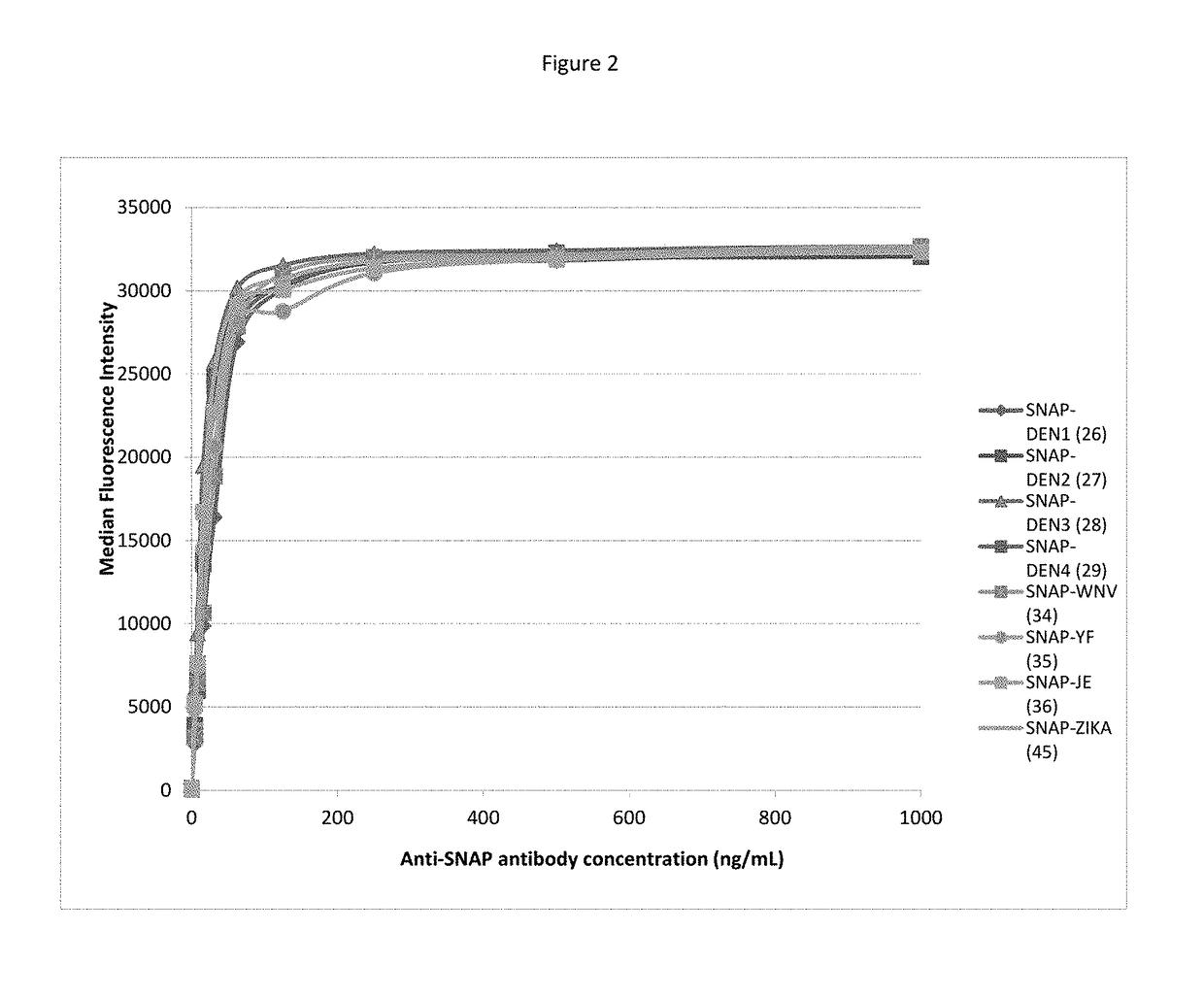
ActiveUS20170276672A1Planar/plate-like light guidesBiological testingDisease surveillanceImmunoprecipitation
The present invention provides kits and assay methods for the early detection of pathogens, precise identification of the etiologic agent, and improved disease surveillance. More specifically, the present invention discloses an immunoassay leading to the rapid and simultaneous detection of antibodies to a wide range of infectious pathogens in biological fluids of infected patients. This immunoassay involves the covalent and oriented coupling of fusion proteins comprising an AGT enzyme and a viral antigen on an identifiable solid support (e.g. fluorescent microspheres), said support being previously coated with an AGT substrate. This coupling is mediated by the irreversible reaction of the AGT enzyme on its substrate. The thus obtained antigen-coupled microspheres show enhanced capture of specific antibodies as compared to antigen-coupled microspheres produced by standard amine coupling procedures. The methods of the invention possess the ability to multiplex, minimize the amount of biological sample, and have enhanced sensitivity and specificity toward target antibodies as compared with classical ELISA or Radio-Immunoprecipitation assays.
Owner:INST PASTEUR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com