Peroxiredoxin 1 binding protein and application thereof
A protein-binding technology, applied in the field of medicine and biology, can solve problems such as reducing the incidence of oral cancer, and achieve the effect of improving the expression of binding proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1P
[0040] The preliminary identification of embodiment 1Prx1 binding protein
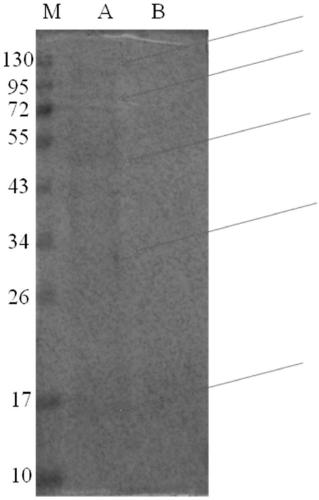
[0041] Co-immunoprecipitation (Co-IP) combined with SDS-PAGE was used to detect the Prx1-binding protein in untreated DOK cells and DOK cells treated with 1 μmol / L nicotine for 7 days. The specific operation was as follows:
[0042] (1) Place a 100mm cell culture dish on ice, wash it once with cold PBS, pour off the PBS, add 1ml of pre-cooled Lysis Buffer, scrape off the cells with a cell scraper, collect the cells, and transfer them to a pre-cooled 2ml centrifuge pipe.
[0043] (2) Add 10 μl of 100× protease inhibitor and phosphatase inhibitor to the cells collected in step 1), place on ice for 40 minutes, and vortex every 10 minutes.
[0044] (3) Centrifuge at 15,300 rpm for 40 min at 4°C, collect the supernatant to obtain soluble whole protein, and place it on ice for later use.
[0045] (4) Prepare the whole cell protein, adjust it to 1 μg / μl, add antibody (Ab) to 500 μl, place on a vortexer at 1...
Embodiment 2
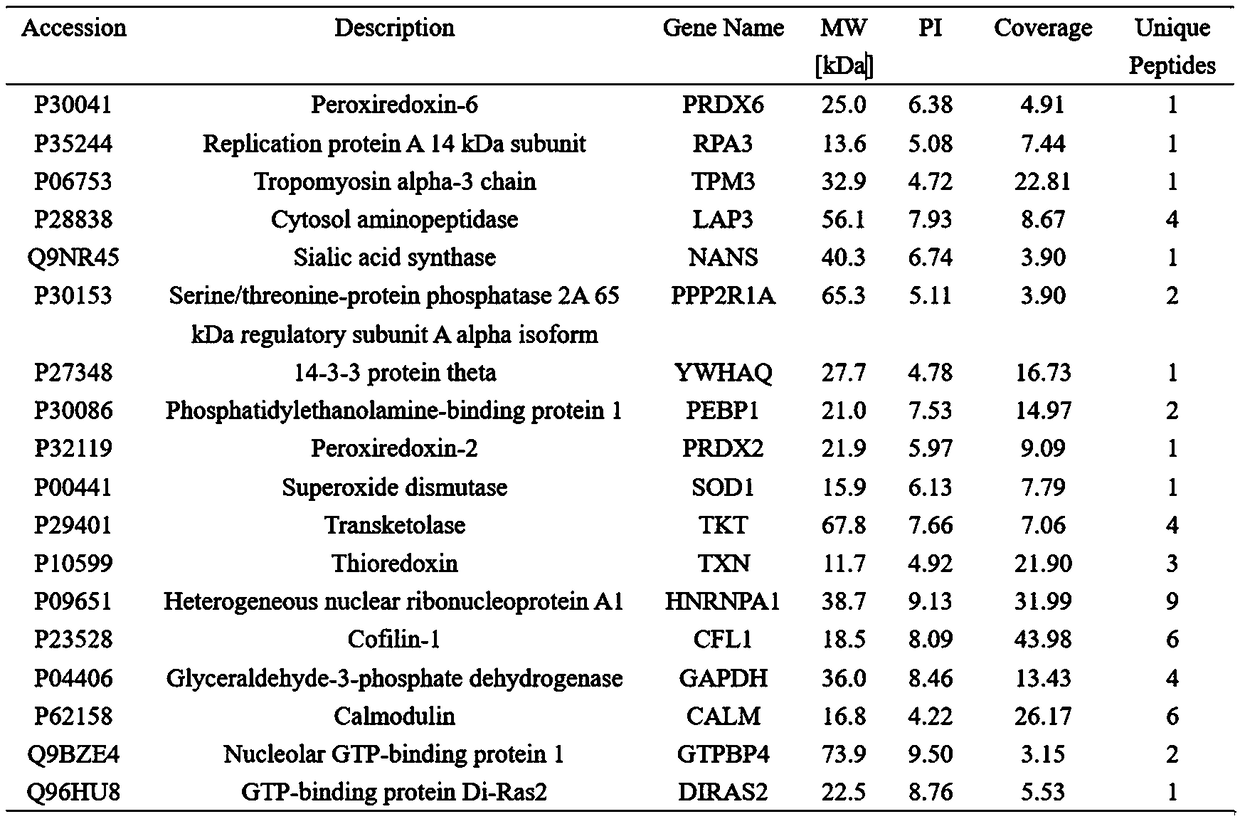
[0053] Example 2 Using bioinformatics methods to analyze Prx1 binding proteins
[0054] The Prx1 binding protein that is detected in embodiment 1 is carried out bioinformatics analysis, mainly comprises GO analysis, kegg pathway analysis, PPI protein interaction network analysis ( Figure 4 ).
[0055] (1) GO analysis is a means to detect the significant function of differential genes based on the gene annotation database. The specific description is as follows.
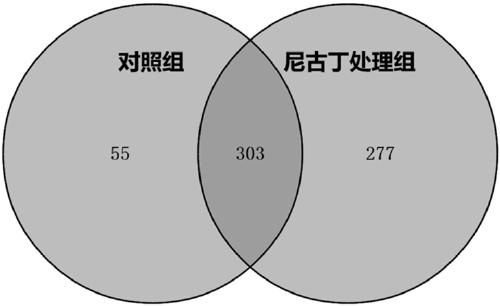
[0056] ① The genes unique to the nicotine treatment group and genes shared by the two groups were respectively annotated by GO based on the database from three levels of biological process, molecular function, and cellular components.
[0057] ② All the GOs in which the genes participated were obtained by the above operation, and the significance level (P-Value) of each GO was calculated by using the Fisher test, so as to screen out the significant GOs with gene enrichment.
[0058] ③ Significant P-Value<0.05 is ma...
Embodiment 3
[0072] Example 3 Validates the screened Prx1-binding protein
[0073] It mainly includes two aspects: using co-immunoprecipitation (Co-IP) combined with Western Blot method to verify the selected binding protein; using immunohistochemical method to detect the expression of the verified binding protein in mouse tongue tissue from the protein level .
[0074] (1) Use Co-IP combined with Western Blot method to verify the screened binding protein Co-IP experiment is carried out as follows:
[0075] ① Wash the cells twice with pre-cooled PBS, and collect the cells with a scraper.
[0076] ② Add 1ml co-immunoprecipitation lysate (containing PMSF, PIC) and lyse on ice for 15min.
[0077] ③Centrifuge at 4°C, 14 000r / min (centrifugal radius 9.5cm) for 15min, and take the supernatant.
[0078] ④The protein concentration was measured using the Bradford method protein quantification kit, and 25 μg of total protein was retained.
[0079] ⑤ Divide the remaining supernatant equally into Pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com