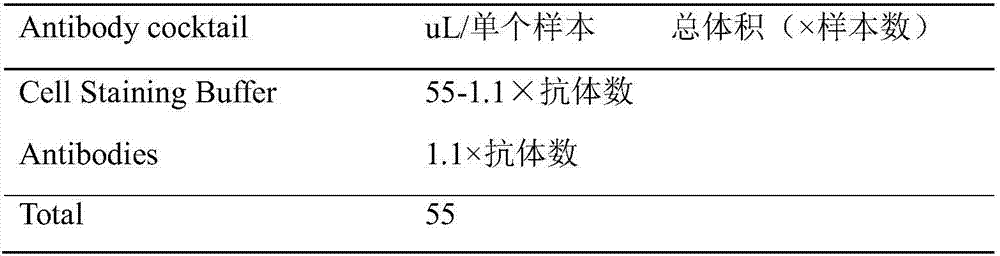
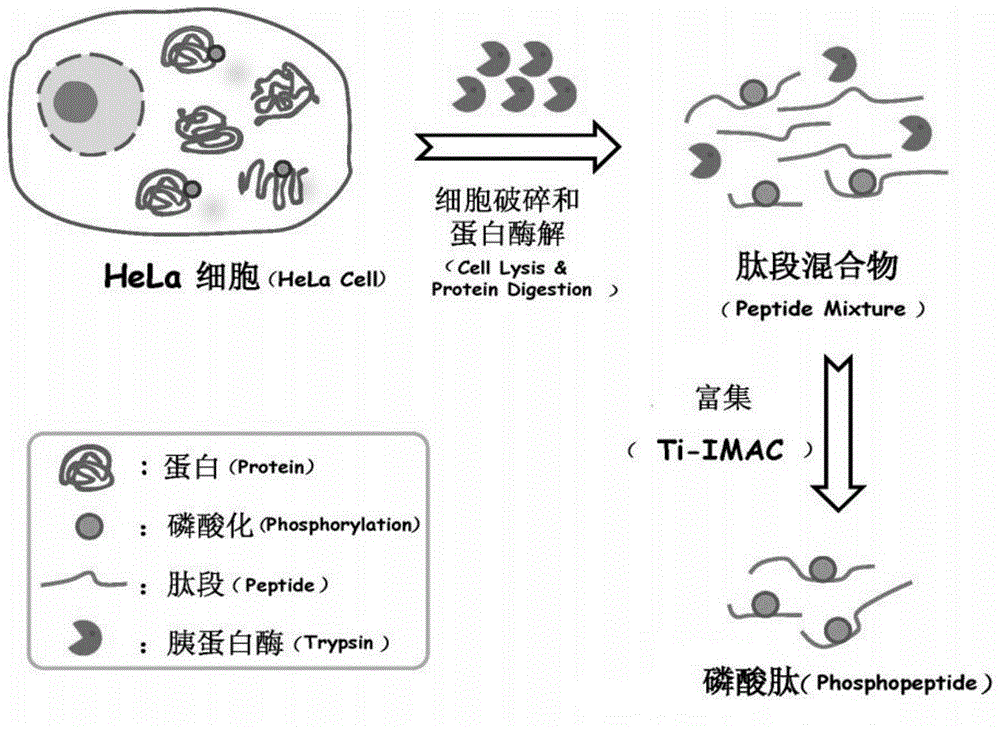
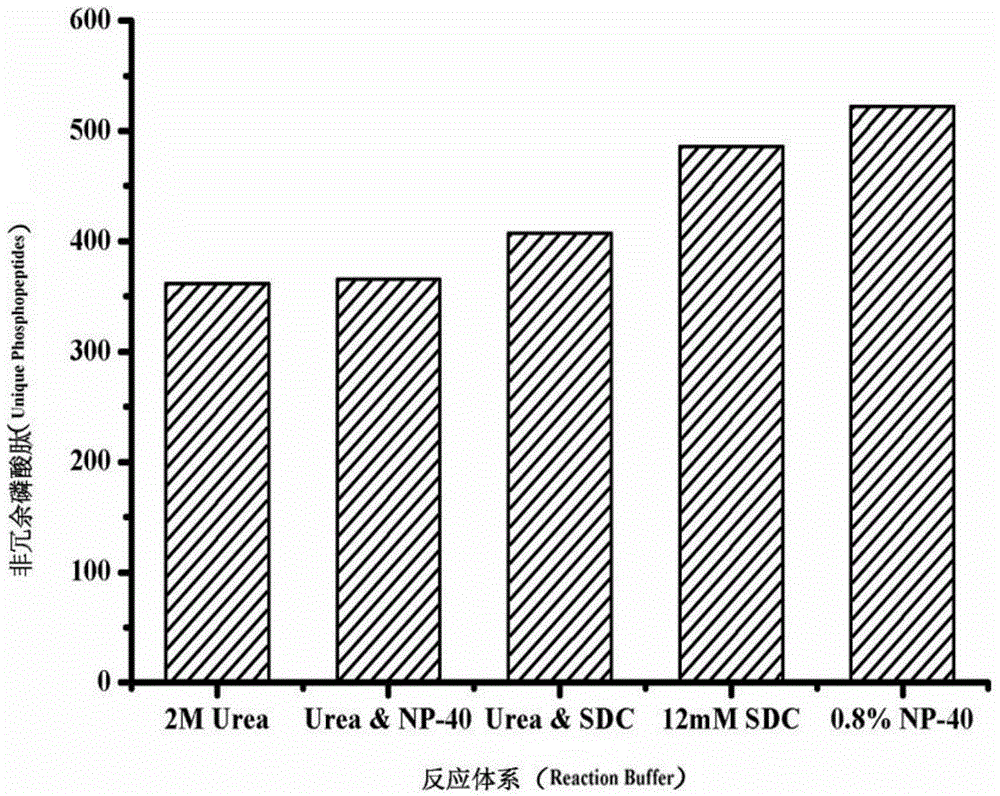
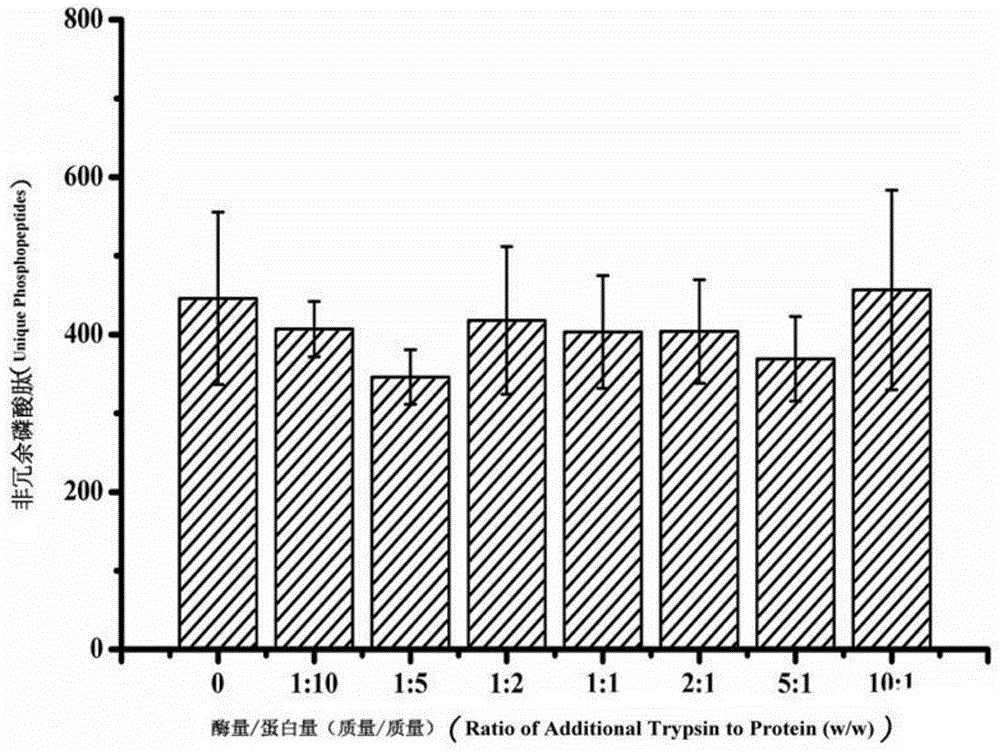
Patents
Literature
196 results about "Phosphorylated proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protein phosphorylation is the attachment of a phosphate (PO 4) group to a protein. The new phosphorus group alters the role of the protein: it can activate, deactivate, or cause a change in function. Protein phosphorylation is fairly common in cells of prokaryotic and eukaryotic organisms.
Cell fixation and use in phospho-proteome screening
InactiveUS7326577B2Expand accessEasy screeningWithdrawing sample devicesChemiluminescene/bioluminescenceEpitopeWilms' tumor
Owner:LAB OF AMERICA HLDG
Systems, methods and kits for characterizing phosphoproteomes
InactiveUS20050164324A1Quick filterImprove developmentMicrobiological testing/measurementIsotope separationPhosphorylationSystems approaches
The invention provides systems, software, methods and kits for detecting and / or quantifying phosphorylatable polypeptides and / or acetylated polypeptides in complex mixtures, such as a lysate of a cell or cellular compartment (e.g., such as an organelle). The methods can be used in high throughput assays to profile phosphoproteomes and to correlate sites and amounts of phosphorylation with particular cell states.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Analyzing phosphorylated proteins
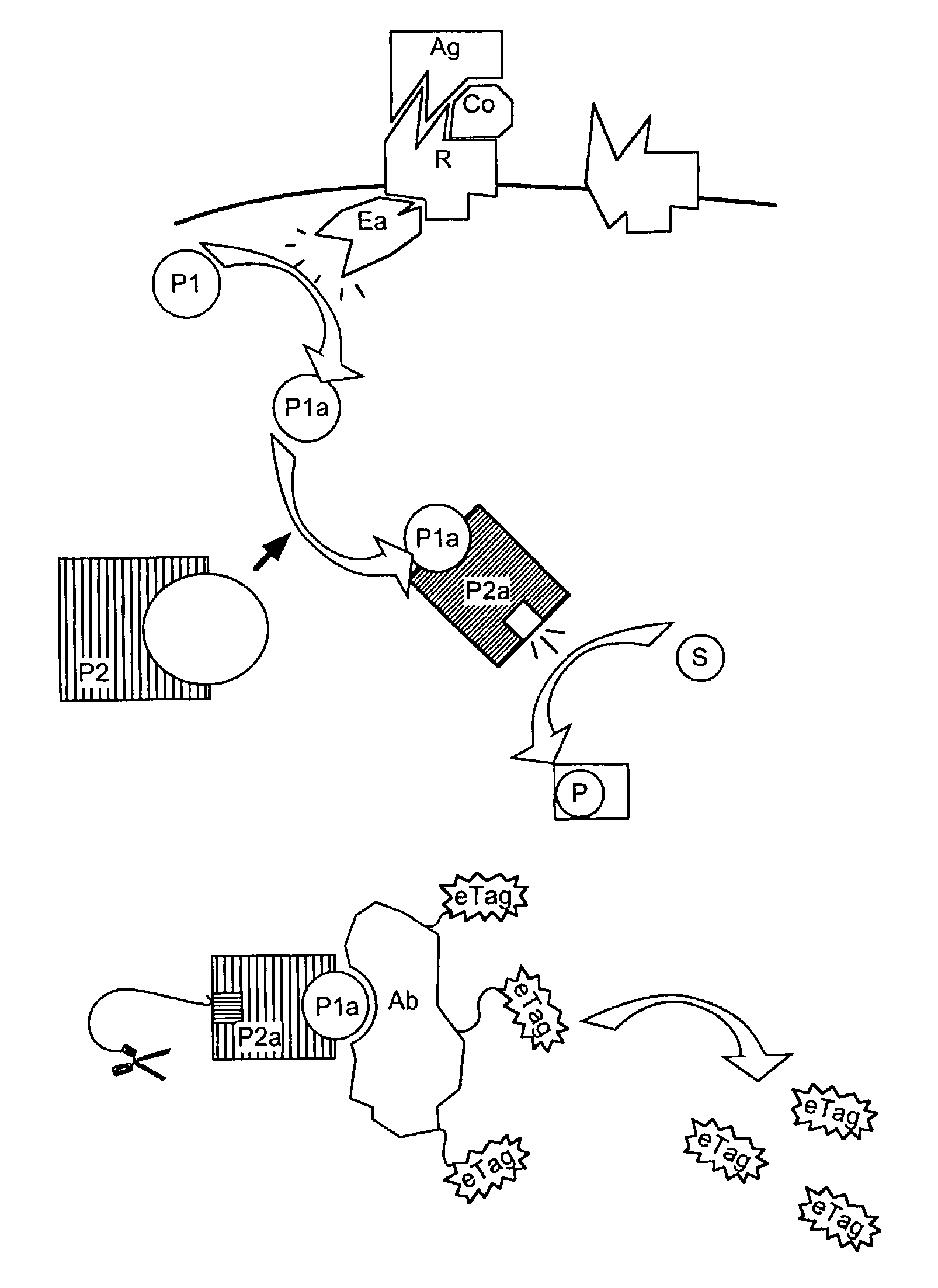
Methods, compositions and kits are disclosed for determining one or more target polypeptides in a sample where the target polypeptides have undergone phosphorylation. A mixture comprising the sample and a first reagent comprising a cleavage-inducing moiety and an IMAC resin for a binding site on a target polypeptide is subjected to conditions under which binding of respective binding moieties occurs. The binding site is the result of phosphorylation activity involving the target polypeptide. The method may be employed to determine the target polypeptide itself. In another embodiment the presence and / or amount of the target polypeptide is related to the presence and / or amount and / or activity of an agent such as an enzyme involved in phosphorylation of the target polypeptide. The interaction between the IMAC resin and the binding site brings the cleavage-inducing moiety into close proximity to a cleavable moiety, which is associated with the polypeptide and is susceptible to cleavage only when in proximity to the cleavage-inducing moiety. In this way, an electrophoretic tag for each of the polypeptides may be released. Released electrophoretic tags are separated and the presence and / or amount of the target polypeptides are determined based on the corresponding electrophoretic tags.
Owner:VIROLOGIC INCORPORATED
Polyclonal antibody of phosphorylating and corresponding non-phosphorylating protein in application for preparing reagent for disease diagnosis, and preparation method
A method for preparing multicloning antibody includes synthesizing polypeptide, connecting the coupling agent with keyhole limpet hemocyanin for forming hapten ¿C carrier protein system, immunizing rabbit then picking up its blood for separating out serum, purifying out multicloning antibody of anti ¿C specific site amino acid phosphorylation protein and anti ¿C corresponding non phosphorylation protein after two steps of antigen affinity chromatography .
Owner:南京川博生物技术有限公司
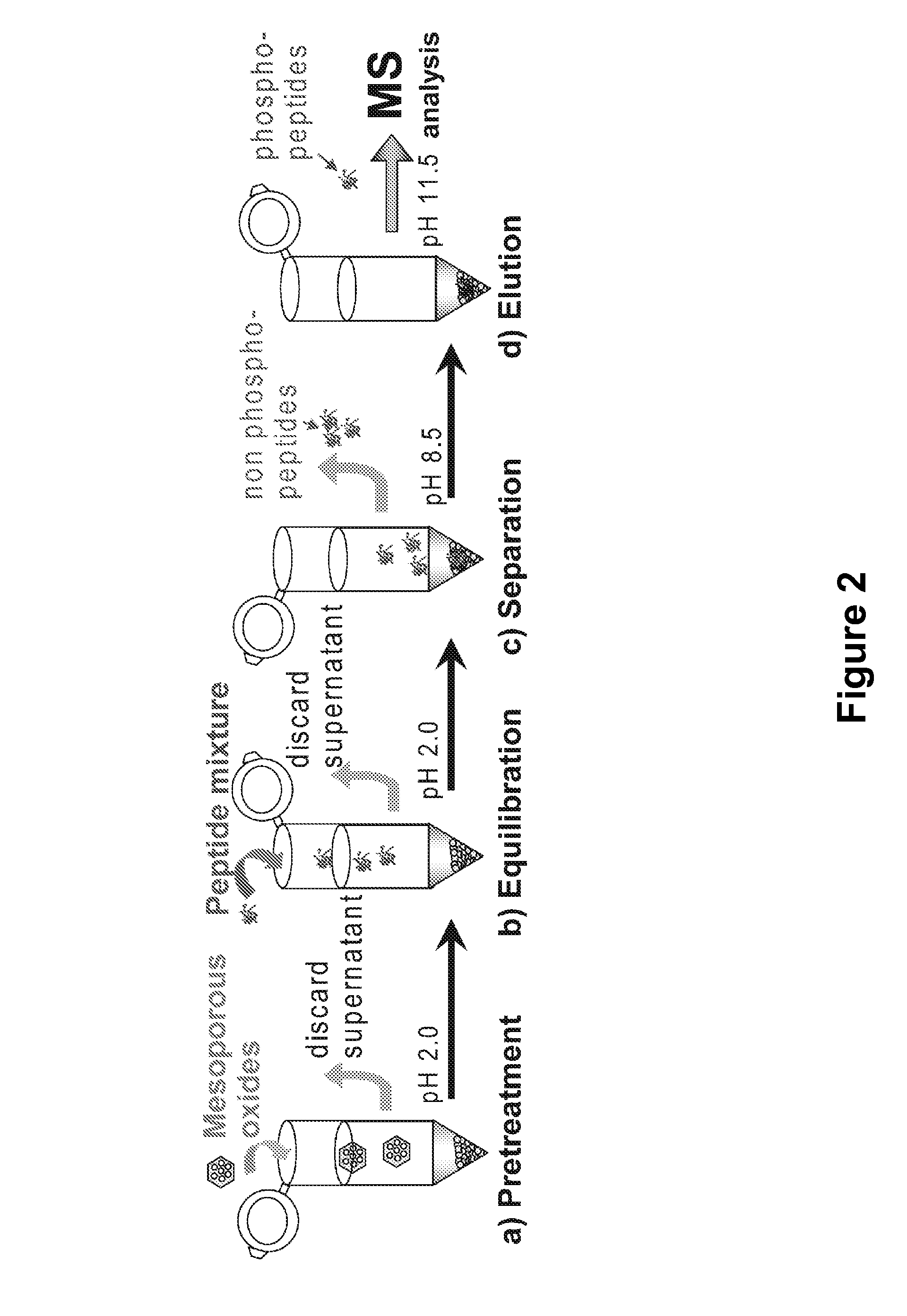
Method for specifically separating and enriching phosphorylated peptide and glycosylated peptide
ActiveCN108440641ALarge specific surface areaGood magnetic responsePeptide preparation methodsProteinPhosphorylated Peptide
The invention provides a method for specifically separating and enriching a phosphorylated peptide and a glycosylated peptide. The method concretely comprises the following steps: preparing a dispersion from a hydrophilic magnetic meso-porous titanium dioxide material, adding the dispersion and a target phosphorylated peptide and glycosylated peptide solution into a sample introduction buffer solution, performing incubation at 37 DEG C for 30-60 min, washing a material with the sample introduction buffer solution, using ammonia water with the volume ratio of 5-20% as an elution buffer solution, performing dot targeting on the obtained eluate, and performing mass spectrometry. The method realizes the simultaneous enrichment of the low-abundance phosphorylated peptide and glycosylated peptide by controlling enriching and eluting conditions, can realize simultaneous large-scale identification of a phosphorylated protein and a glycosylated protein by MALDI-TOF MS or nano-LC-MS / MS, and hasa broad application prospect in post-translational modification proteomics.
Owner:FUDAN UNIV
Selective labeling agent for phosphoproteome analysis and phosphorylated site analysis
InactiveUS20050176093A1High detection sensitivityOrganic active ingredientsPeptide/protein ingredientsPhosphopeptideSlurry
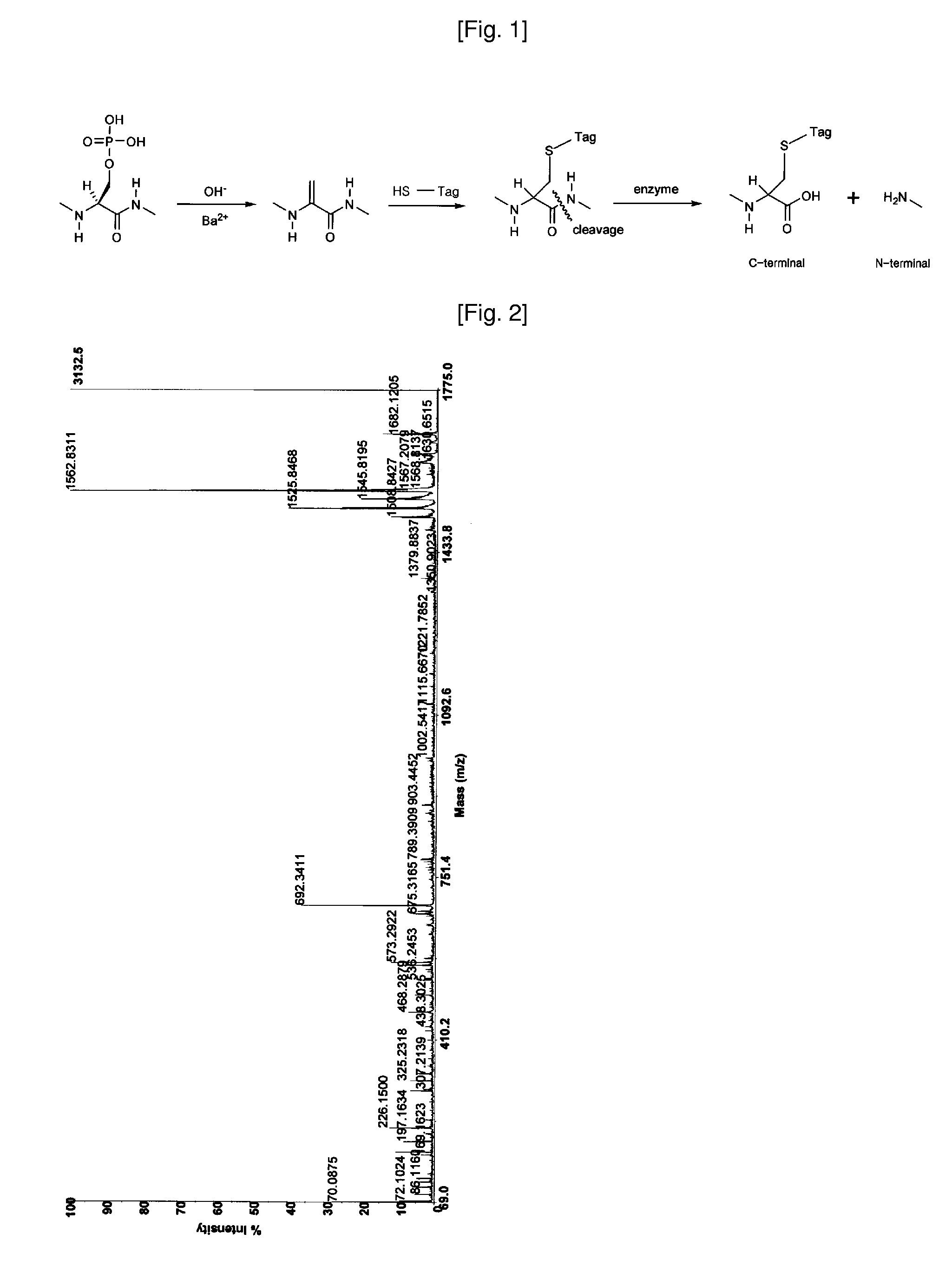
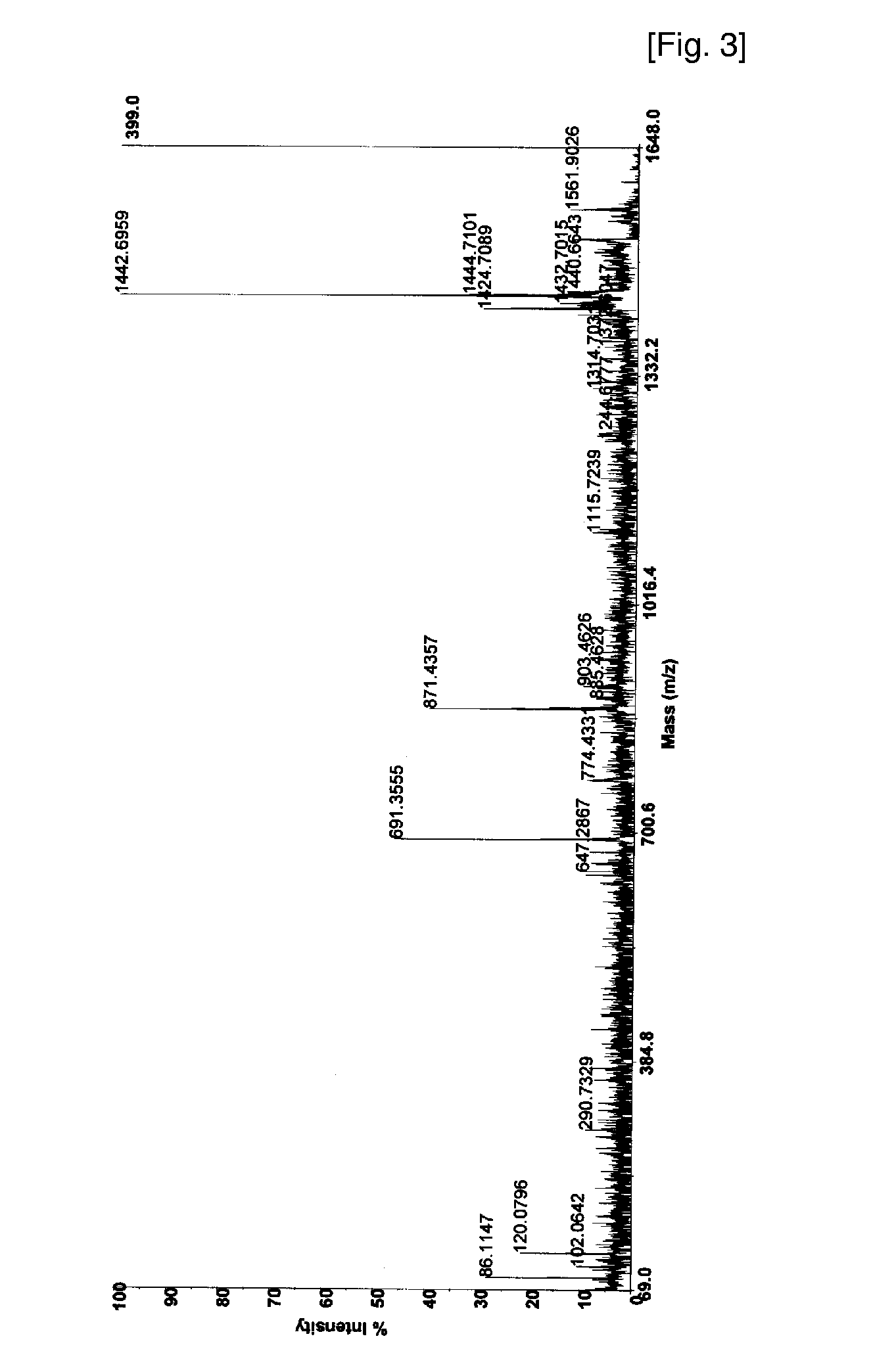
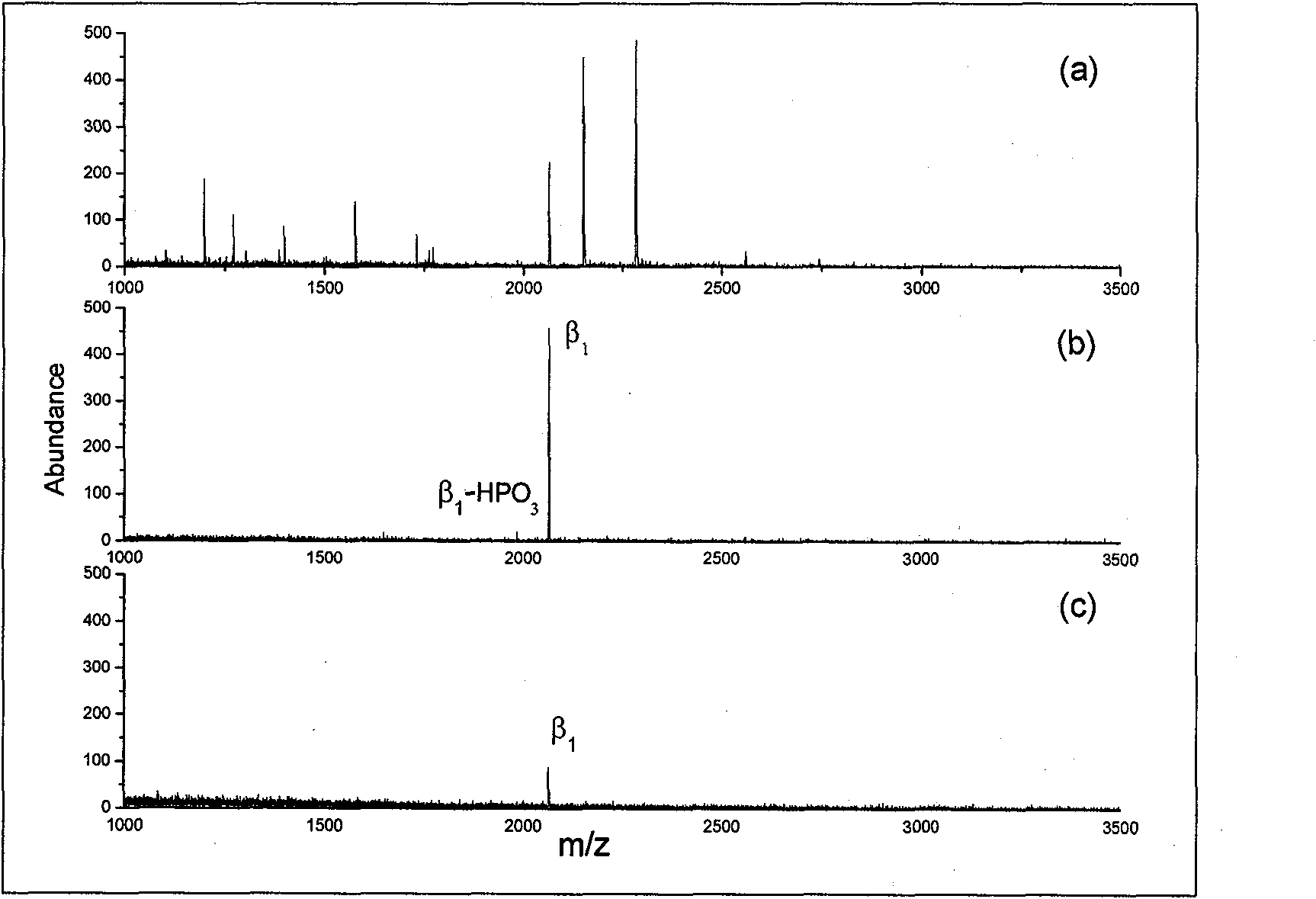
Disclosed is a method of analyzing mass of the phosphoproteins or phosphopeptides and of analyzing phosphorylated positions at a phosphoprotein or phosphopeptide, comprising the steps of: 1) dephosphorylating at least one Ser and / or Thr residue of the phosphoprotein or phosphopeptide; 2) tagging the dephosphorylated amino acid residues with a tag having a R-L-G moiety wherein R is a nucleophilic functional group that selectively bind with dephosphorylated amino acid residues, G is selected from the group consisting of guanidine moiety or protected guanidine moiety such as a mono-N-protected guanidino group, a di-N,N′-protected guanidino group and an N′-protected guanidino group, and L is a linker linking the R and the G; and 3) subjecting the tagged proteins or peptides to mass spectrometry. The method is capable of precisely analyzing mass of phosphoproteins of trace amounts as well as positions of phosphoryated amino acids.
Owner:KOREA BASIC SCI INST
Method for fast enriching and appraising phosphopeptide on MALDI-TOF-MS sample target
InactiveCN101196527AFast grooming processSimple and fast operationComponent separationBiological testingChemical reactionDesorption
The invention relates to a method of fast enrichment and fast identification on the phosphoeptide of the modificatory sample target by the matrix-assisted laser-desorption / time-of-flight mass spectrometry (MALDI-TOF-MS), which is to physically adsorb the fine magnetic nano particle onto the sample target, wash the sample after the sample is loaded to remove the non phosphoeptide and phosphate, add the matrix to make cocrystallization with the phosphoeptide absorbed on the sample target and then conduct the MALDI-TOF-MS analysis. The method does not relate to the chemical reaction without pre-processing to the target, which can modify the target MALDI made of different substrate materials; A plurality of samples complete their enrichment at the same time on the modified target MALDI; There are no sample transfer in the whole process from the sample loading to the mass spectrometric analysis. The invention is easy, fast and sensitive to operate, which has a good application value in the large-scale analysis of the phosphoproteome.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
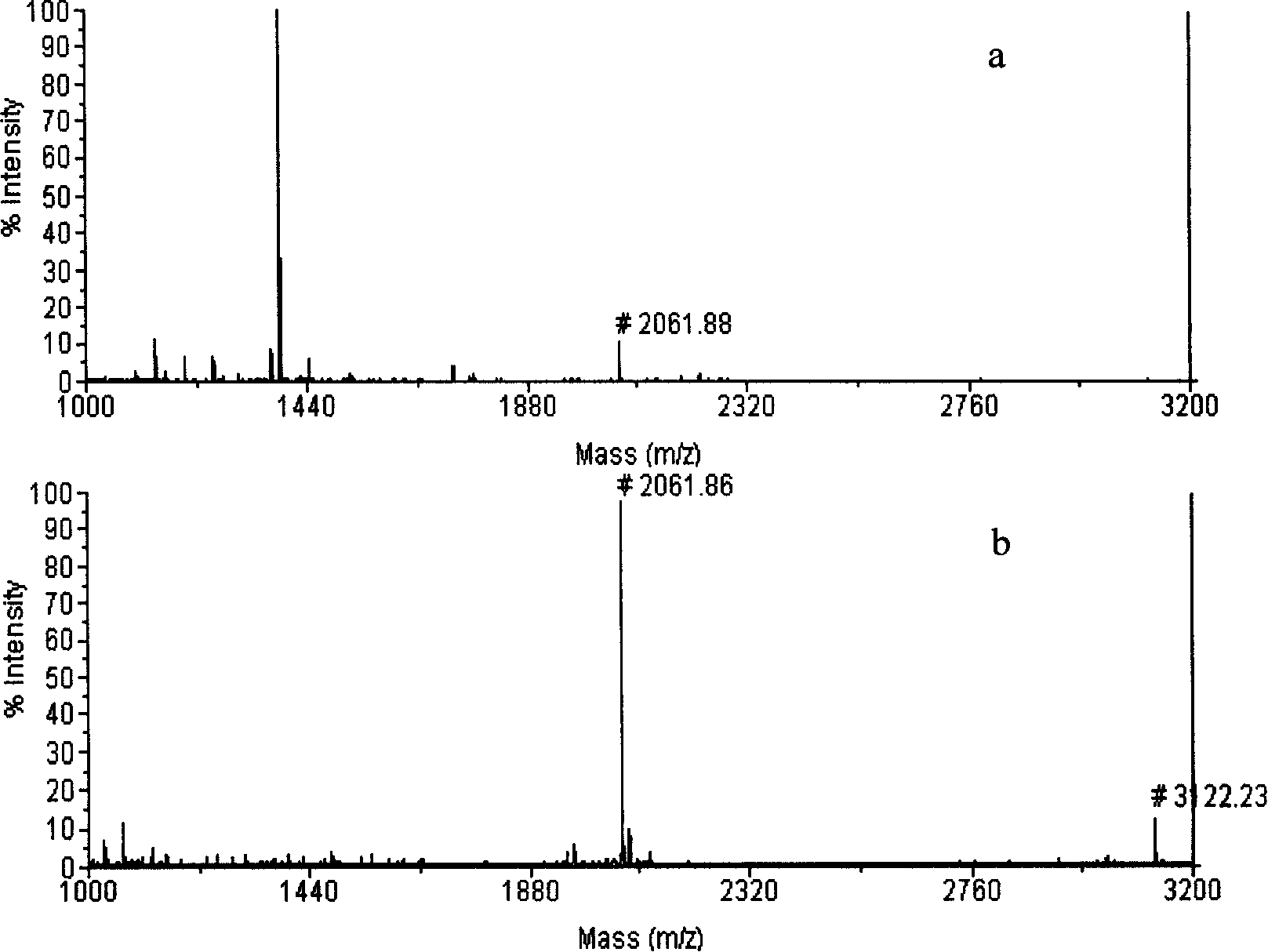
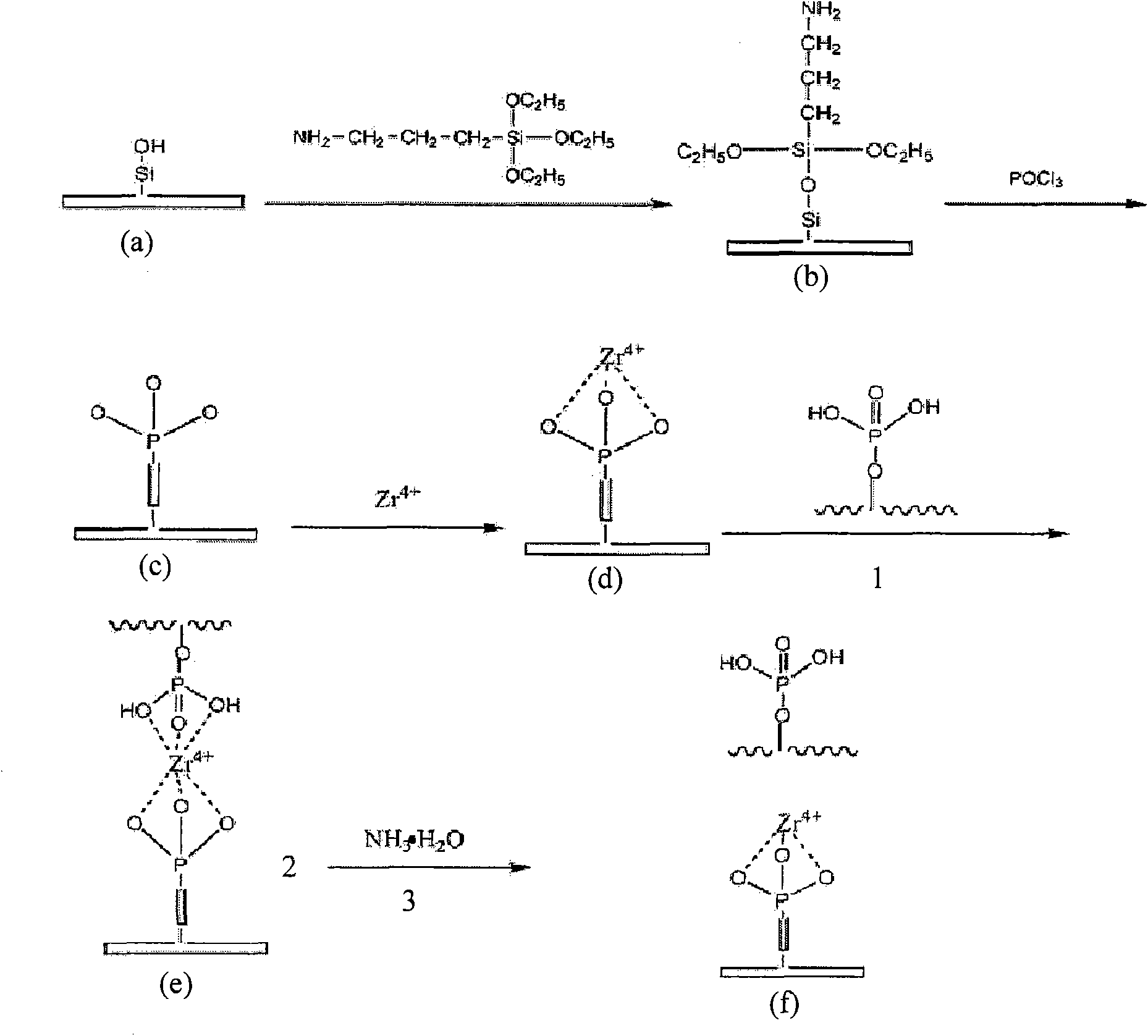
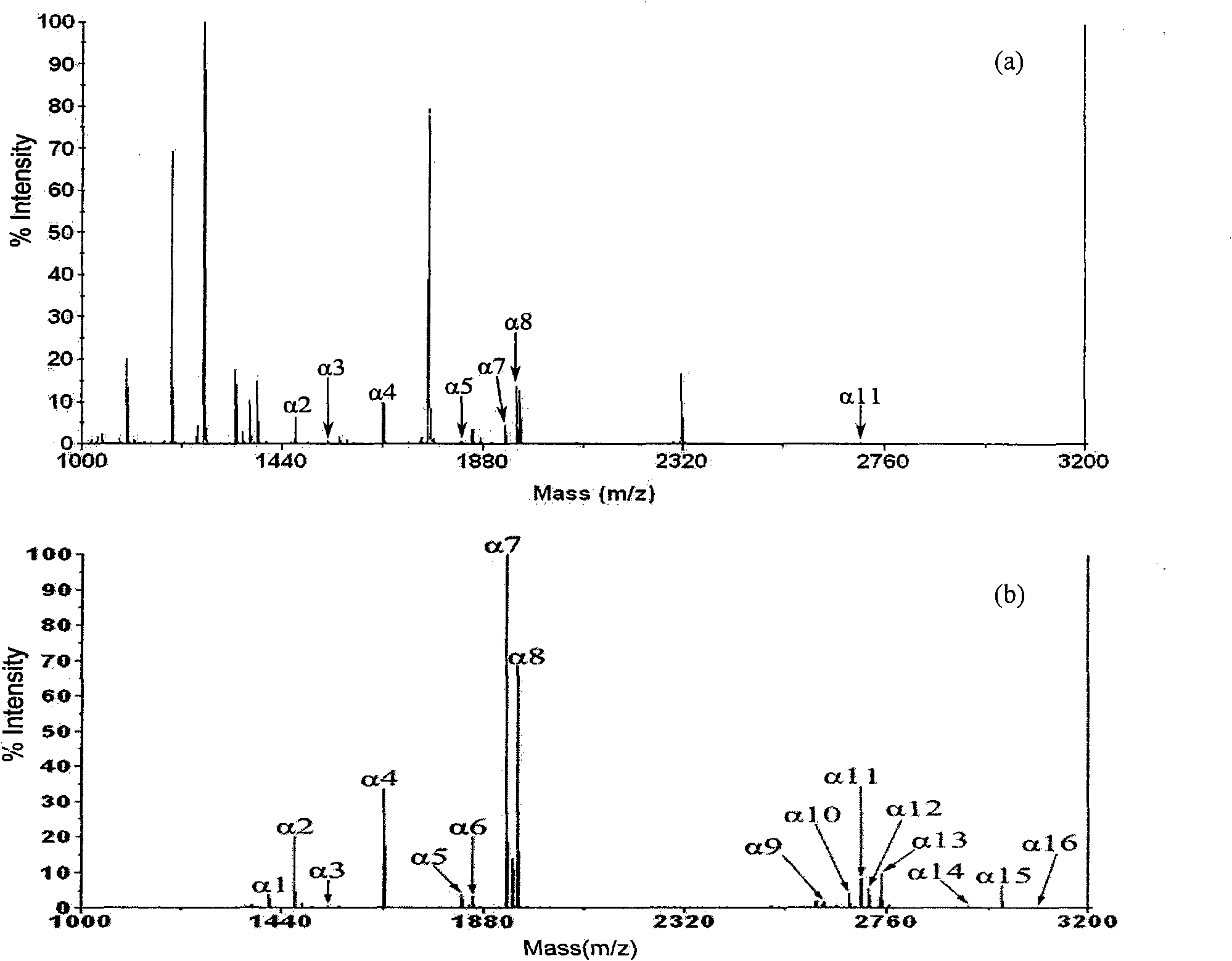
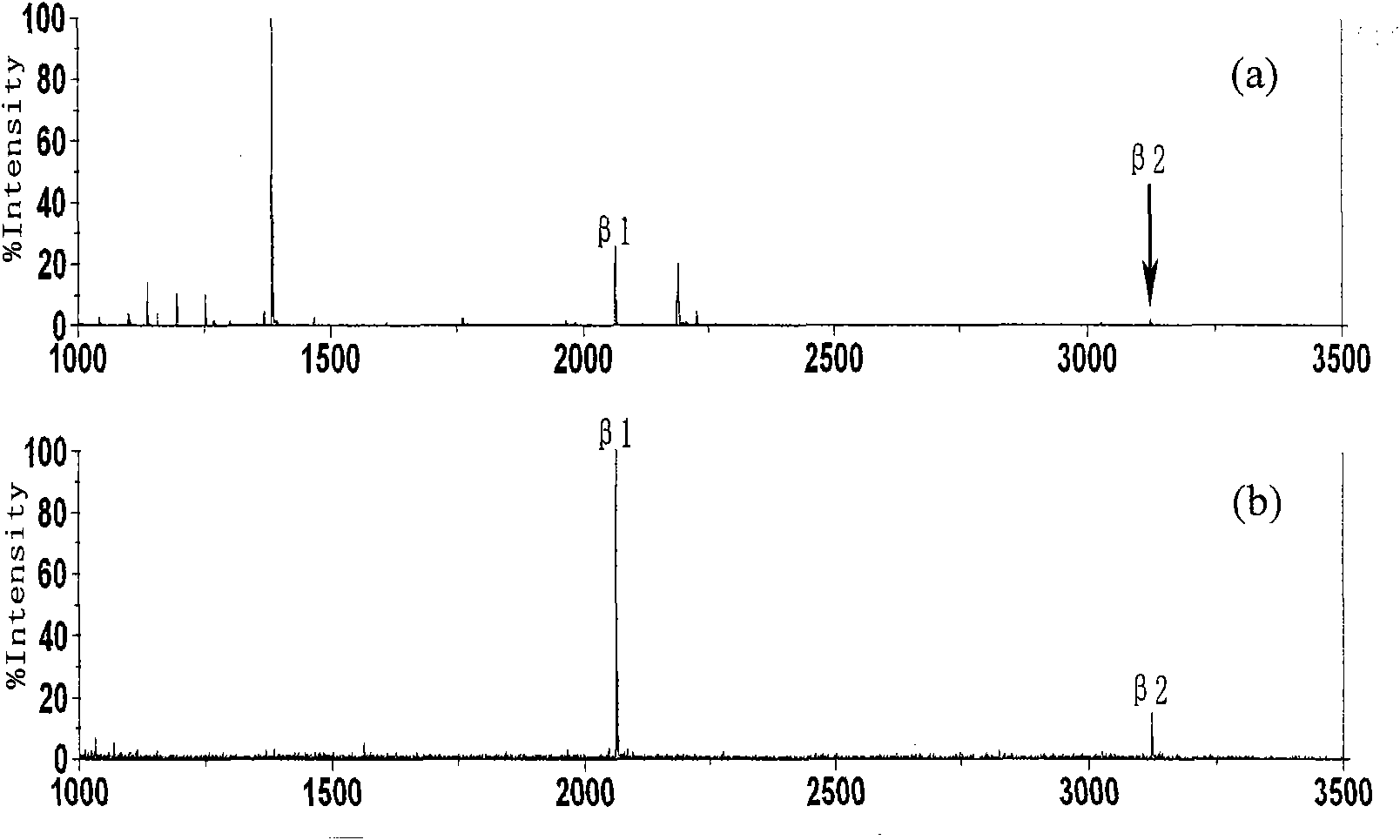
Open-tubular capillary column enriching phosphoeptide or phosphorylated protein and method
InactiveCN101685051ARealize automatic online enrichment analysisMeet analysis needsComponent separationPreparing sample for investigationPhosphorylationProtein insertion
The invention relates to a method for enriching phosphoeptide or phosphorylated protein in an off-line way and an on-line way for a functionally modified open-tubular capillary column, which comprisesthe following steps: preparing an open-tubular capillary column, an inner wall of which can selectively adsorb the zirconium phosphate functional group of phosphoeptide or phosphorylated protein, enriching the phosphoeptide or phosphorylated protein after loading samples, and then, using a cleaning buffer solution to remove non-phosphoeptide or non-phosphorylated protein and salt; and after the cleaning process is finished, using eluent to elute the phosphoeptide or phosphorylated protein, wherein the eluted phosphoeptide or phosphorylated protein can be marked on the MALDI target to carry out normal MALDI-TOF-MS analysis, or the eluted phosphoeptide or phosphorylated protein can be inoculated on the liquid phase part of microliter or nanoliter LC-ESI-MS to be combined with ESI-MS / MS to automatically enrich and analyze the phosphoeptide or phosphorylated protein in the on-line way.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Method for enriching and detecting phosphorylated proteins
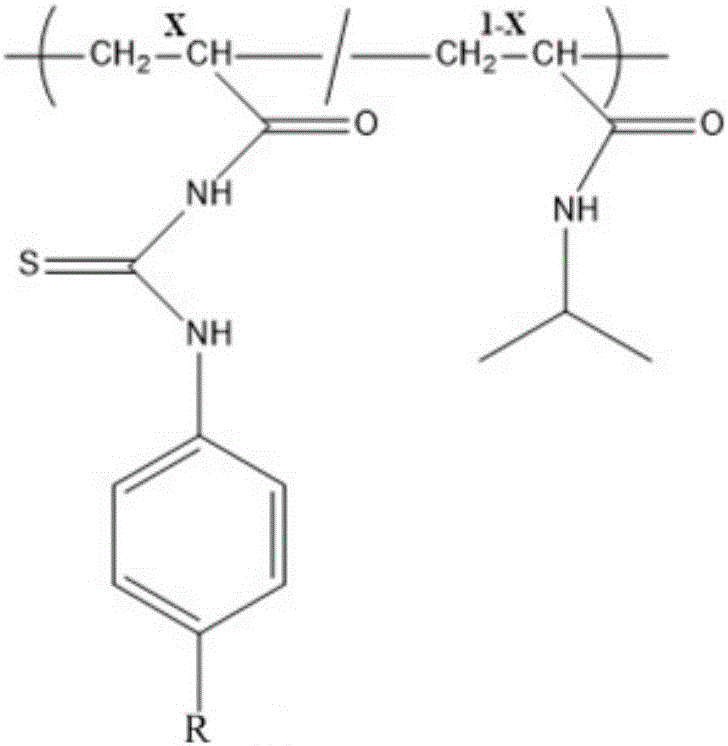
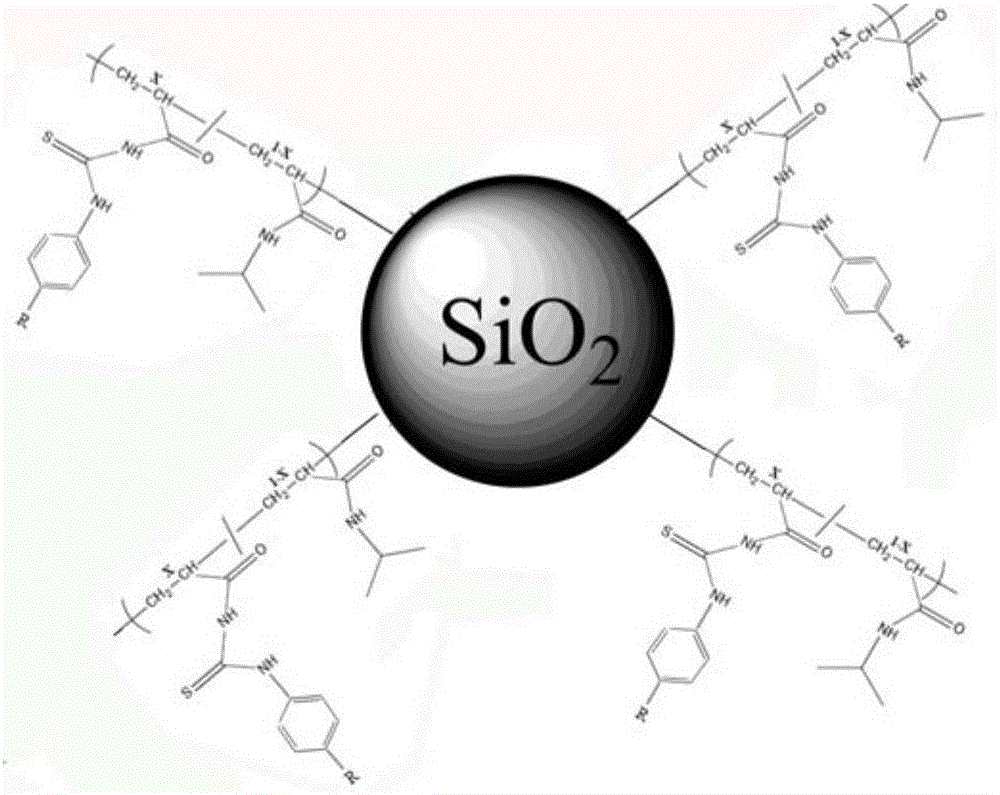
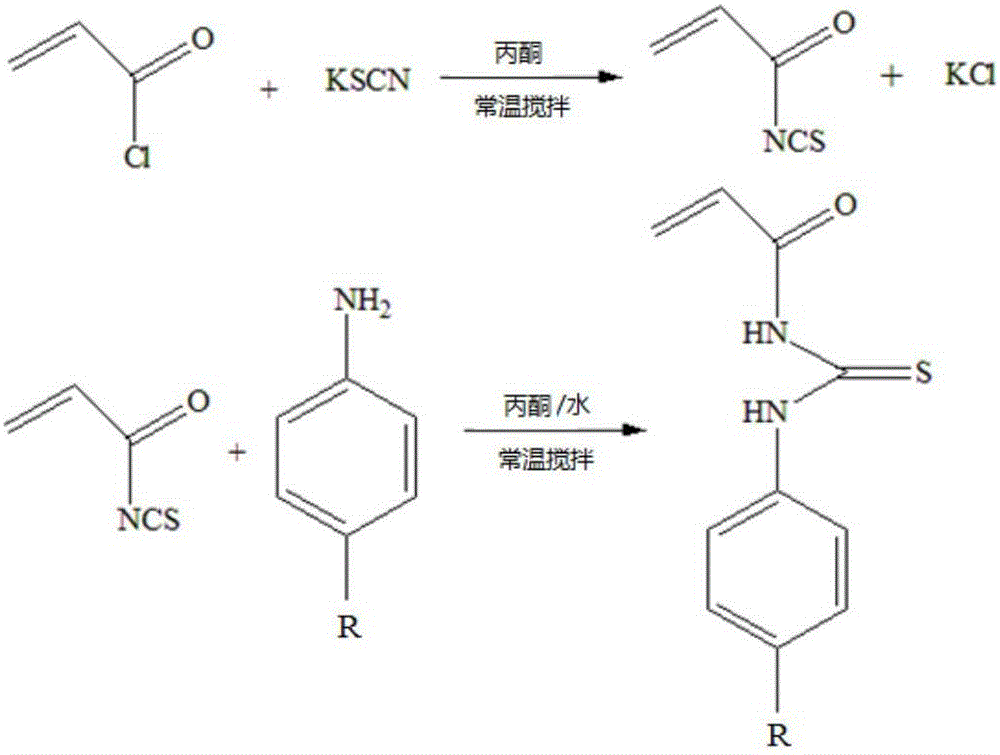
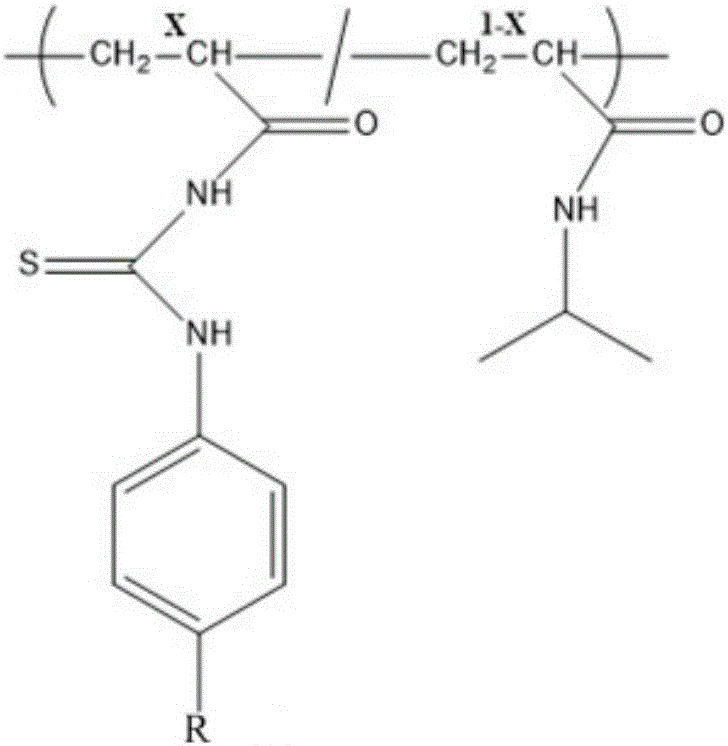
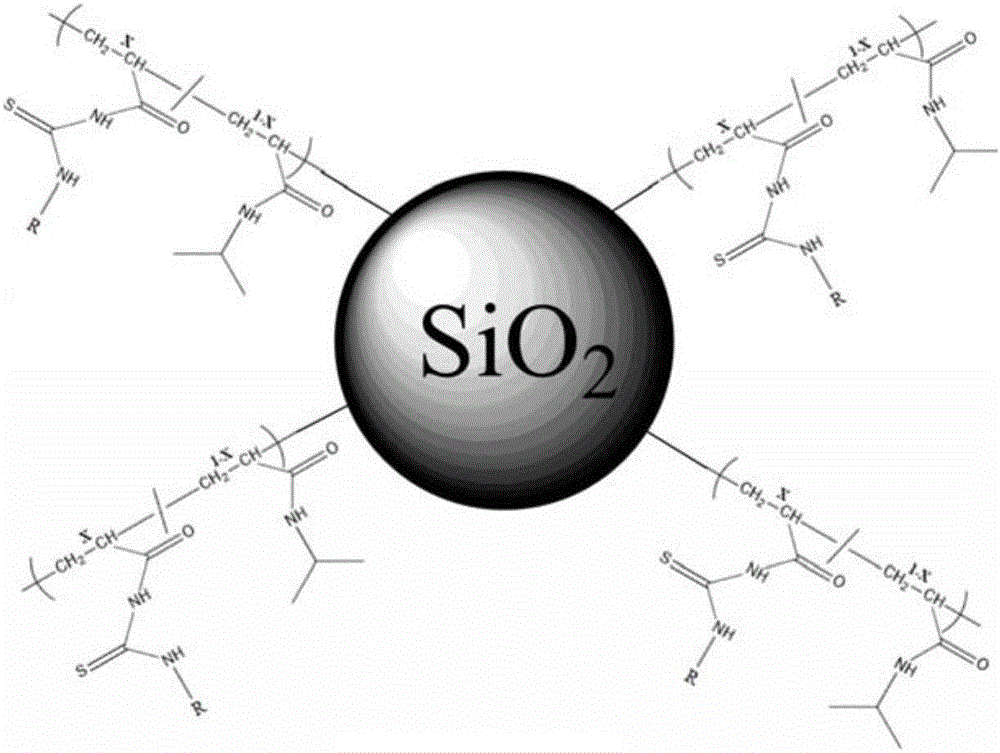
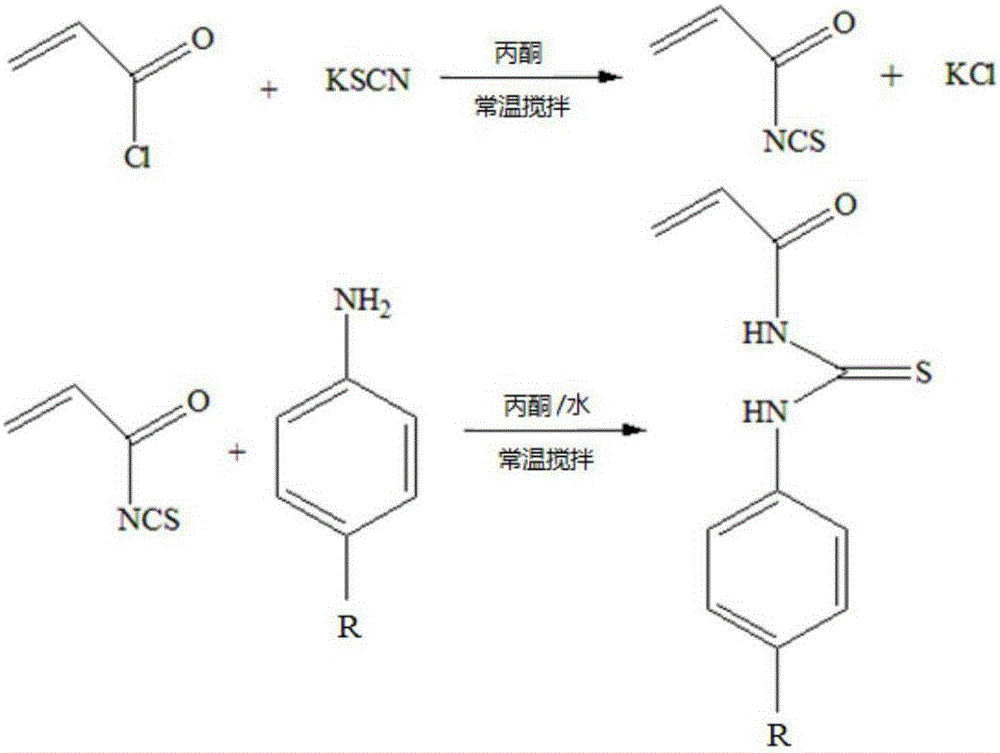
The invention relates to the field of chemical analysis of materials and organic chemistry, and provides a method for enriching and detecting phosphorylated proteins. The method comprises the following steps: directly mixing a protein mixture, which is obtained by mixing phosphorylated proteins and non-phosphorylated proteins, with an enriching material, separating and enriching the non-phosphorylated proteins and the phosphorylated proteins by adopting a dispersive solid phase extraction mode, and carrying out chromatographic analysis on a sample, wherein the enriching material comprises a substrate and a double-component copolymer layer formed on the surface of the substrate; the double-component copolymer layer is 50 to 100 nm in thickness. According to the method, the enriching material and the dispersive solid phase extraction mode are organically combined, so that high-selectivity, high-repetitiveness and high-flux enrichment of the phosphorylated proteins in a complicated mixture can be realized.
Owner:WUHAN UNIV OF TECH
Sample pretreatment method based on flow combination ICP-MS (Inductively Coupled Plasma Mass Spectrometry) single cell protein detection
ActiveCN107314965AImpact AnalysisImpact statementMaterial analysis by electric/magnetic meansIndividual particle analysisPretreatment methodPeripheral blood mononuclear cell
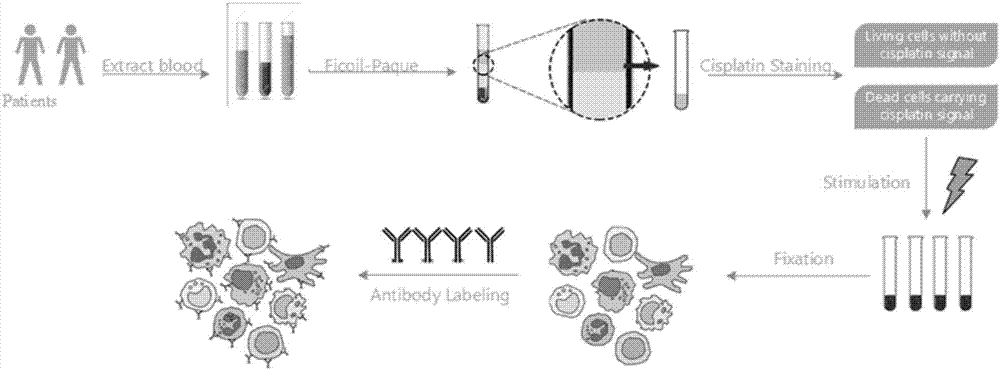
The invention discloses a sample pretreatment method based on flow combination ICP-MS (Inductively Coupled Plasma Mass Spectrometry) single cell protein detection, and belongs to the technical field of sample pretreatment of flow cytometry. The sample pretreatment method comprises the following steps: (1) collecting and transporting a whole blood sample; (2) performing PBMC (Peripheral Blood Mononuclear Cell) cell separation; (3) performing cell active dyeing; (4) performing cell stimulation; (5) performing cell immobilization; (6) performing surface antibody dyeing; (7) performing intracellular phosphorylated protein dyeing; (8) performing single cell labeling, and the like. With the combination of a metal element labeled antibody with cell surface antigen, labeled cells are mixed with beads as internal reference for standardization, dead cells and living cells are distinguished in the pretreatment process, then the situation that the testing result analysis and description are affected by too many dead cells is avoided, single cells are distinguished from cell dimmers, cell trimers or even cell multimers, and flow combination ICP-MS single cell protein detection requirements can be very well met.
Owner:马鞍山普梅森医学检验实验室有限公司
Rapid treatment method for phosphoproteome sample
InactiveCN104949864AQuick analysisPromote lysisPreparing sample for investigationHigh concentrationPretreatment method
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Hydrophilic metal ion immobilization affinity magnetic bead and preparation and application thereof
ActiveCN102760543AImprove hydrophilicityGood choiceNanomagnetismPeptide preparation methodsMagnetic beadAdenosine
The invention relates to a preparation method of a hydrophilic metal ion immobilization affinity magnetic bead, which comprises the following steps: the surface of an amino magnetic bead is subjected to amino activation through glutaral; the activated magnetic bead is connected with an adenosine disodium triphosphate molecule through a surface modification reaction; the surface of the activated magnetic bead is bonded with a hydrophilic functional molecule containing a plurality of phosphate and hydroxyl radicals, so as to be beneficial for subsequent metal ion immobilization; at last, the immobilization of a metal ion is realized in a solution containing the metal ion; and the preparation of the hydrophilic metal ion immobilization affinity magnetic bead is accomplished. The hydrophilic metal ion immobilization affinity magnetic bead has the advantages that the preparation method is simple; compared with other metal ion immobilization affinity chromatographic columns or magnetic beads, the hydrophilic metal ion immobilization affinity magnetic bead prepared by the method is high in hydrophily, good in selectivity, good in preparation repeatability and stable in performance, and has a good specific concentration ability to phosphorylated peptides; the operation is simple; the complicated centrifugation operation can be avoided; and the hydrophilic metal ion immobilization affinity magnetic bead has wide application prospects in proteomics, especially in phosphorylated proteomics.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for quantifying phosphokinase activity on proteins
InactiveUS7888050B2Microbiological testing/measurementBiological material analysisProtein targetTyrosine
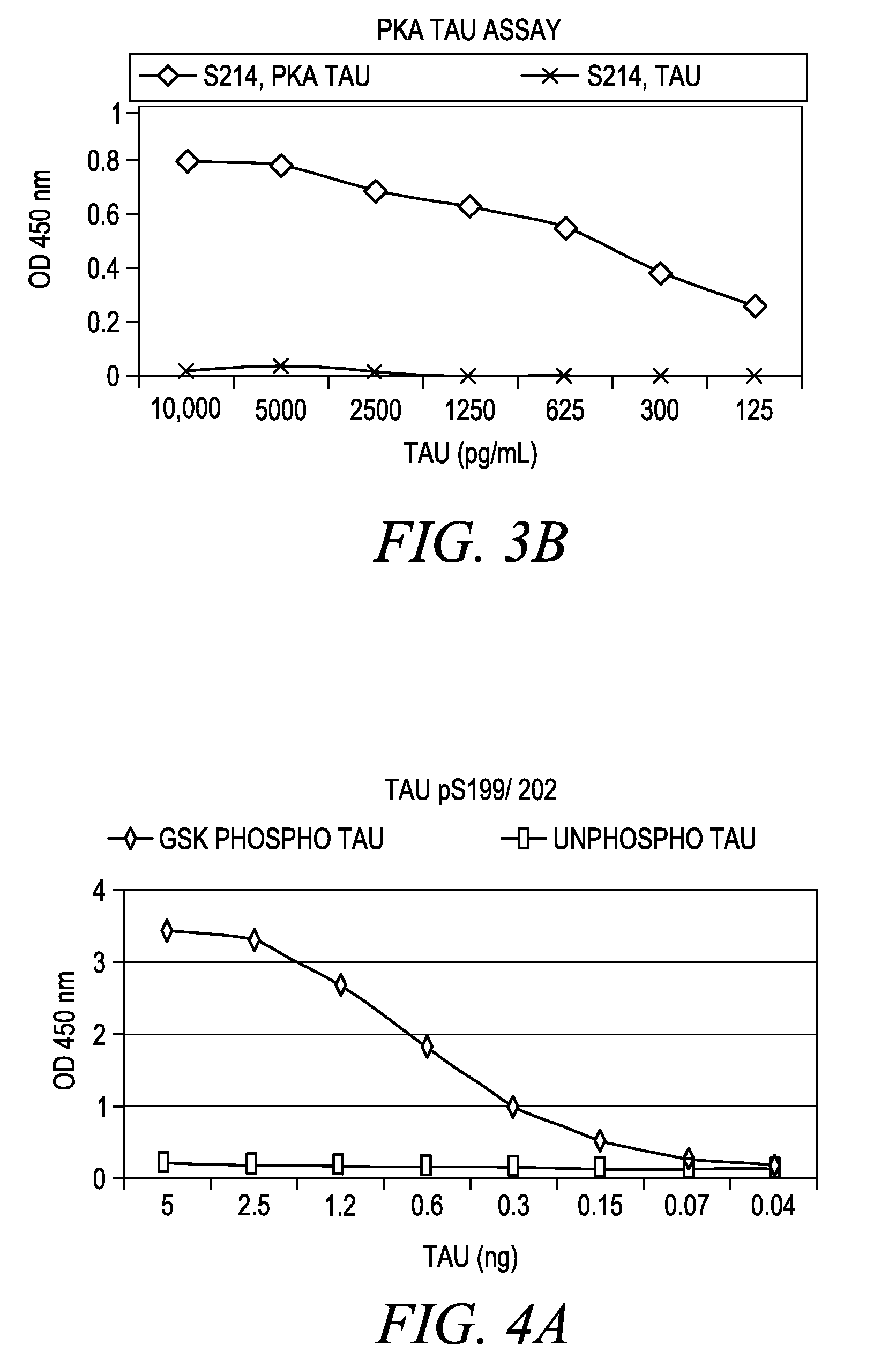
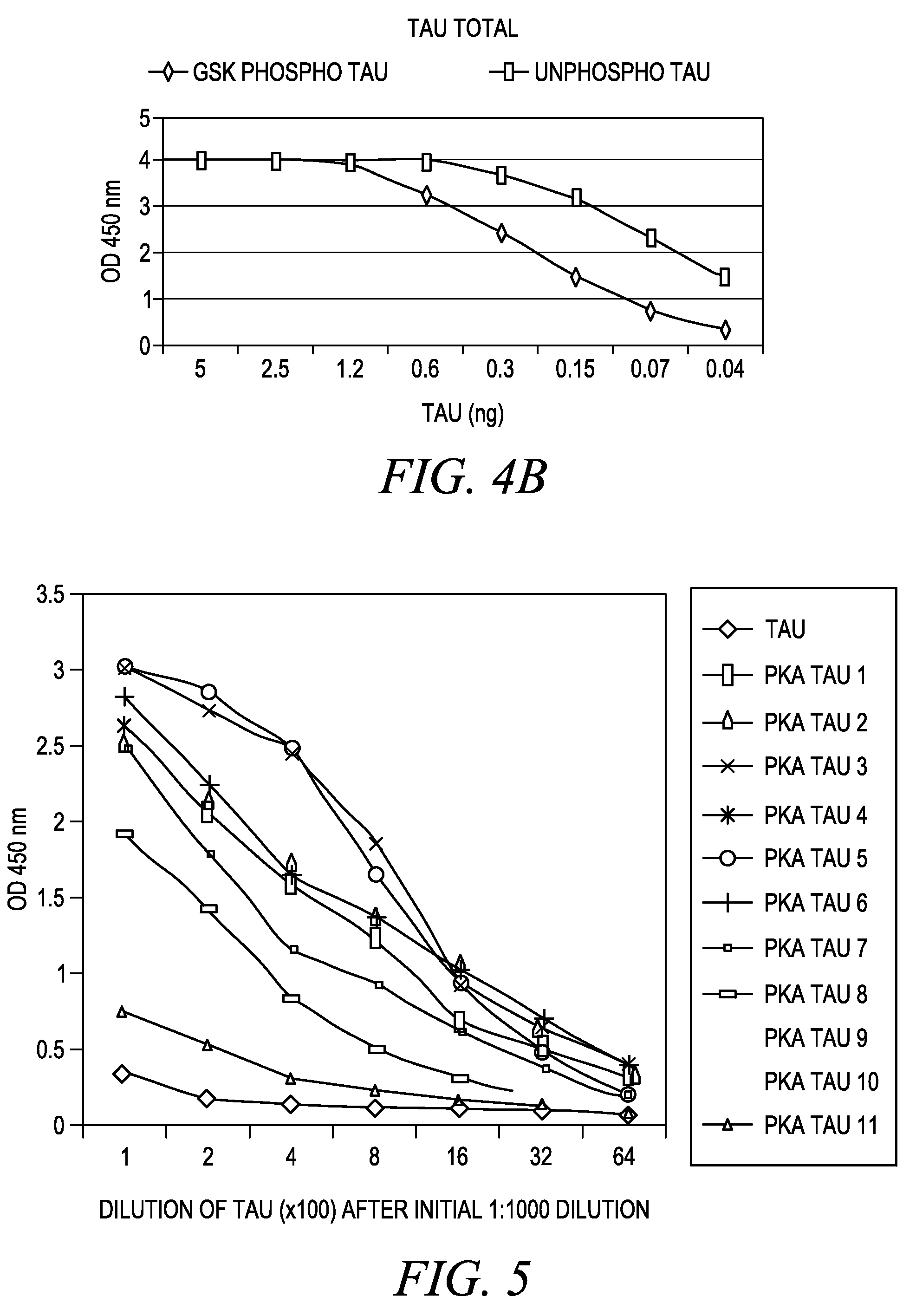
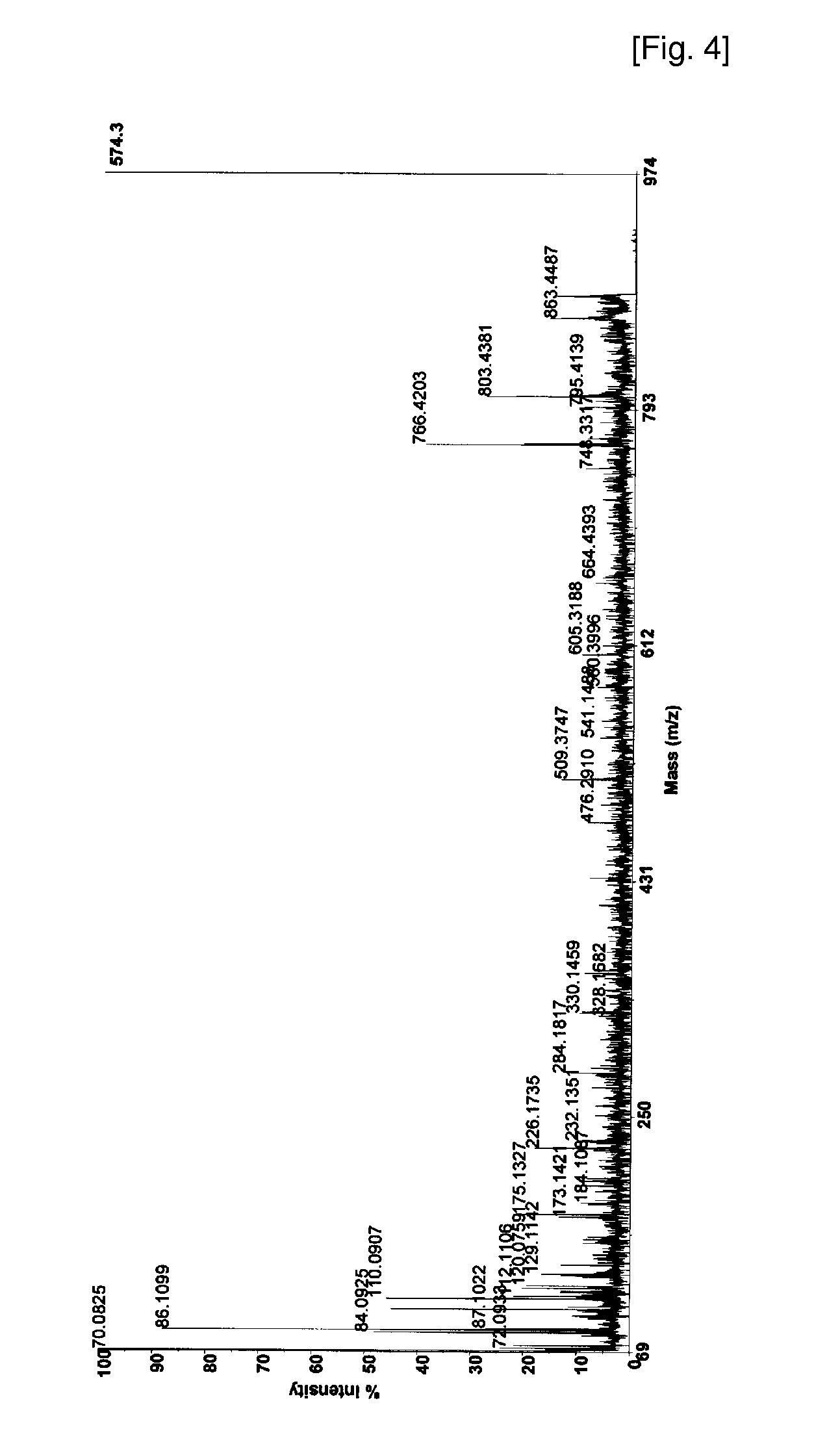
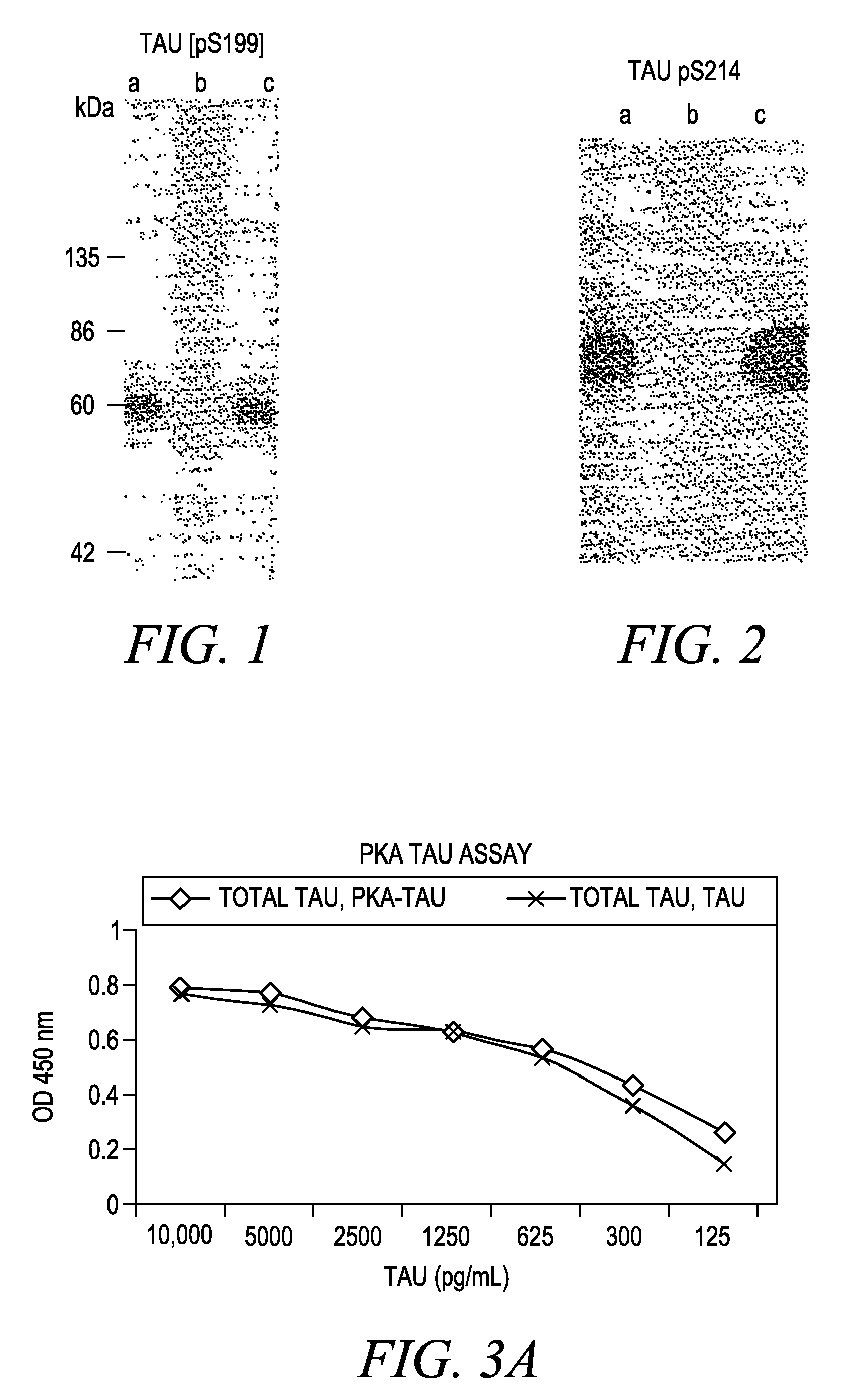
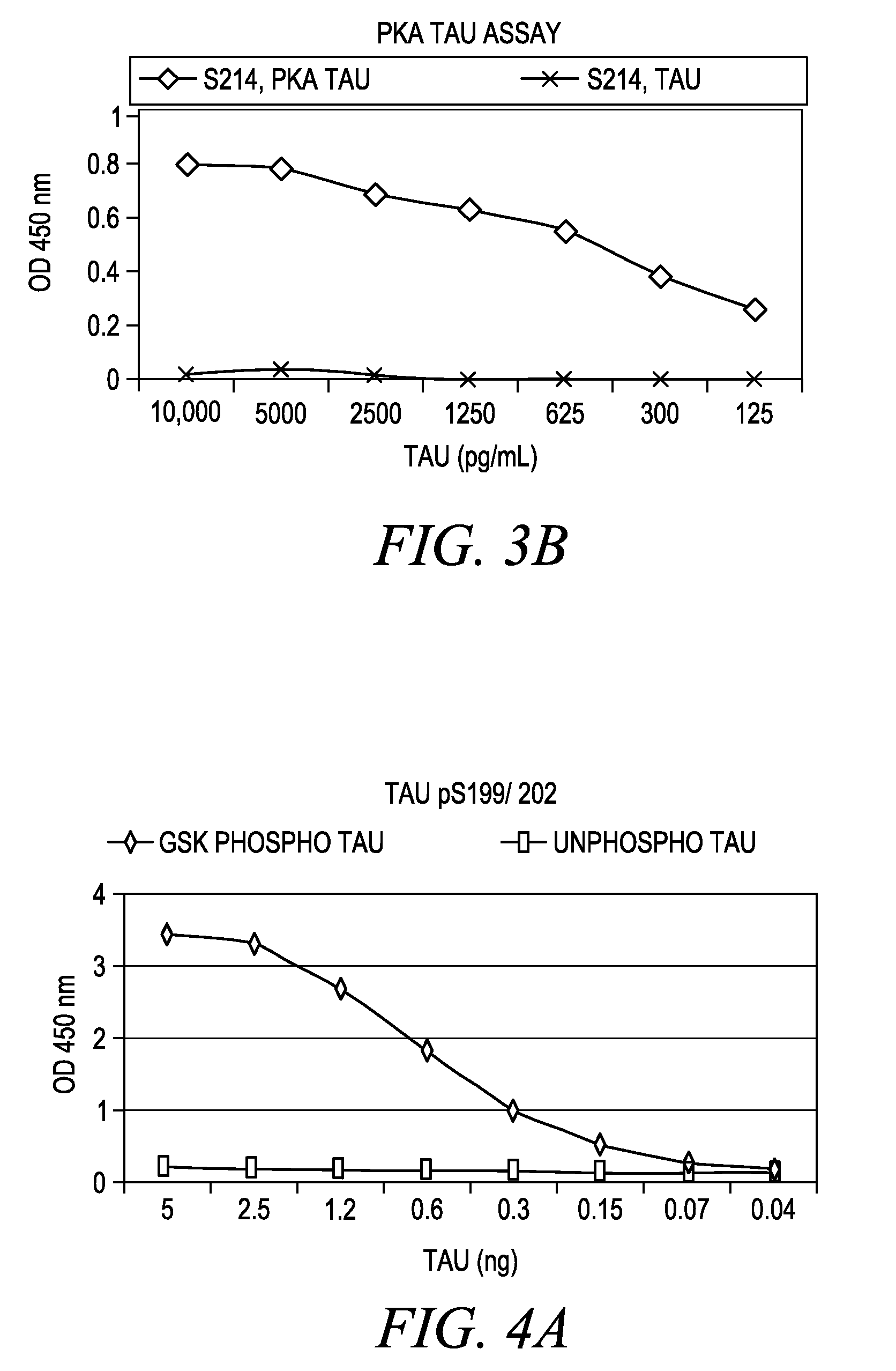
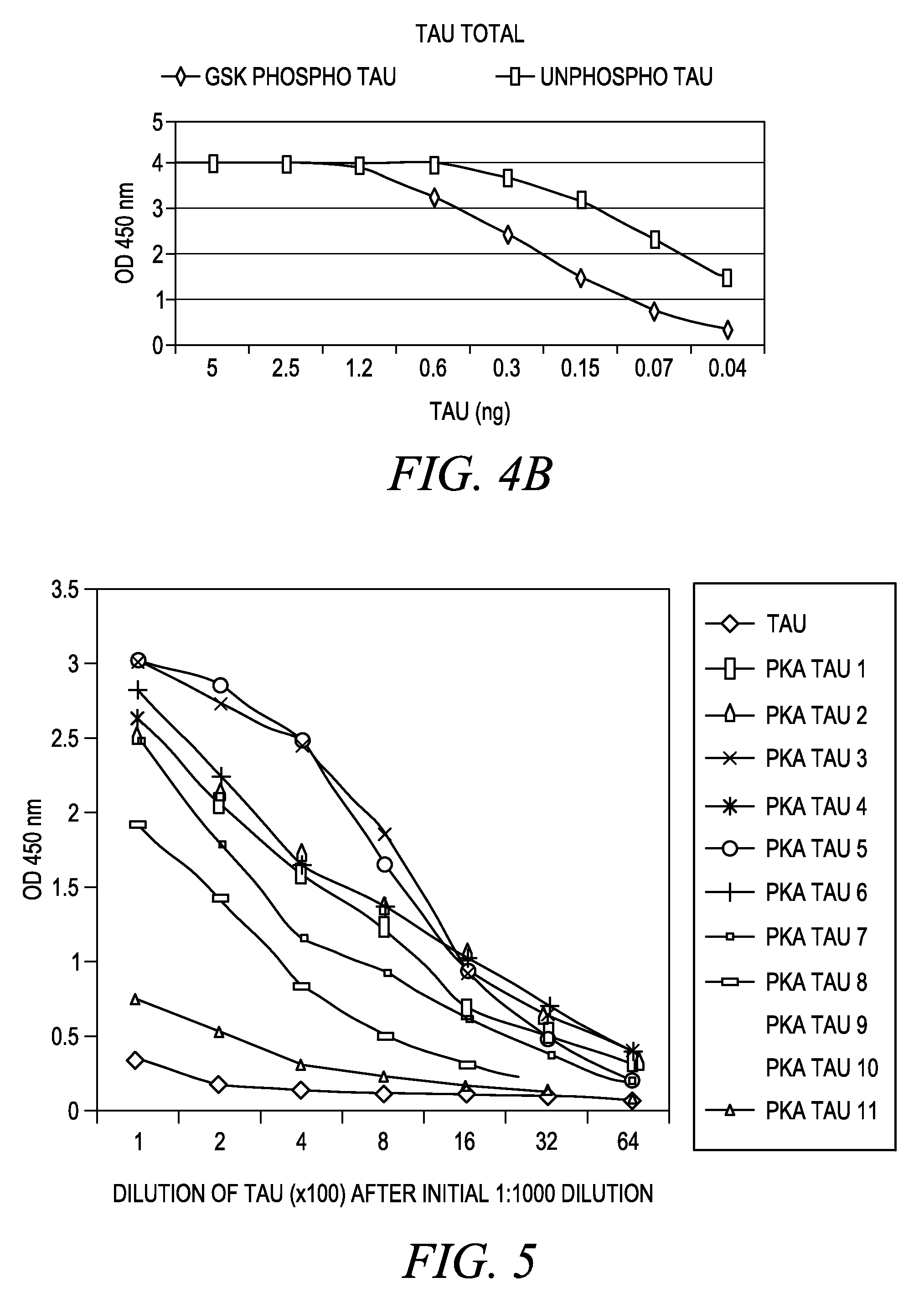
The invention involves a method for measuring phosphorylation of proteins at specific sites and, as such, is an indicator of the protein kinase activity of enzymes capable of phosphorylating those sites. The method involves the in vitro or in vivo phosphorylation of a target protein at a specific serine, threonine or tyrosine residue, subjecting that protein (non-phosphorylated) to reaction mixture containing all reagents, including phosphokinase which allow the creation of a phosphorylated form of protein. The phosphorylated protein is measured by contacting it with an antibody specific for the phosphorylation site(s). The invention includes antibodies useful in practicing the methods of the invention. The invention particularly relates to all proteins modified by phosphorylation and dephosphorylation as illustrated by Tau, Rb and EGFR proteins and antibodies specific for the site of phosphorylation of the Tau, Rb or EGFR proteins.
Owner:LIFE TECH CORP
Mesoporous metal oxide materials for phosphoproteomics
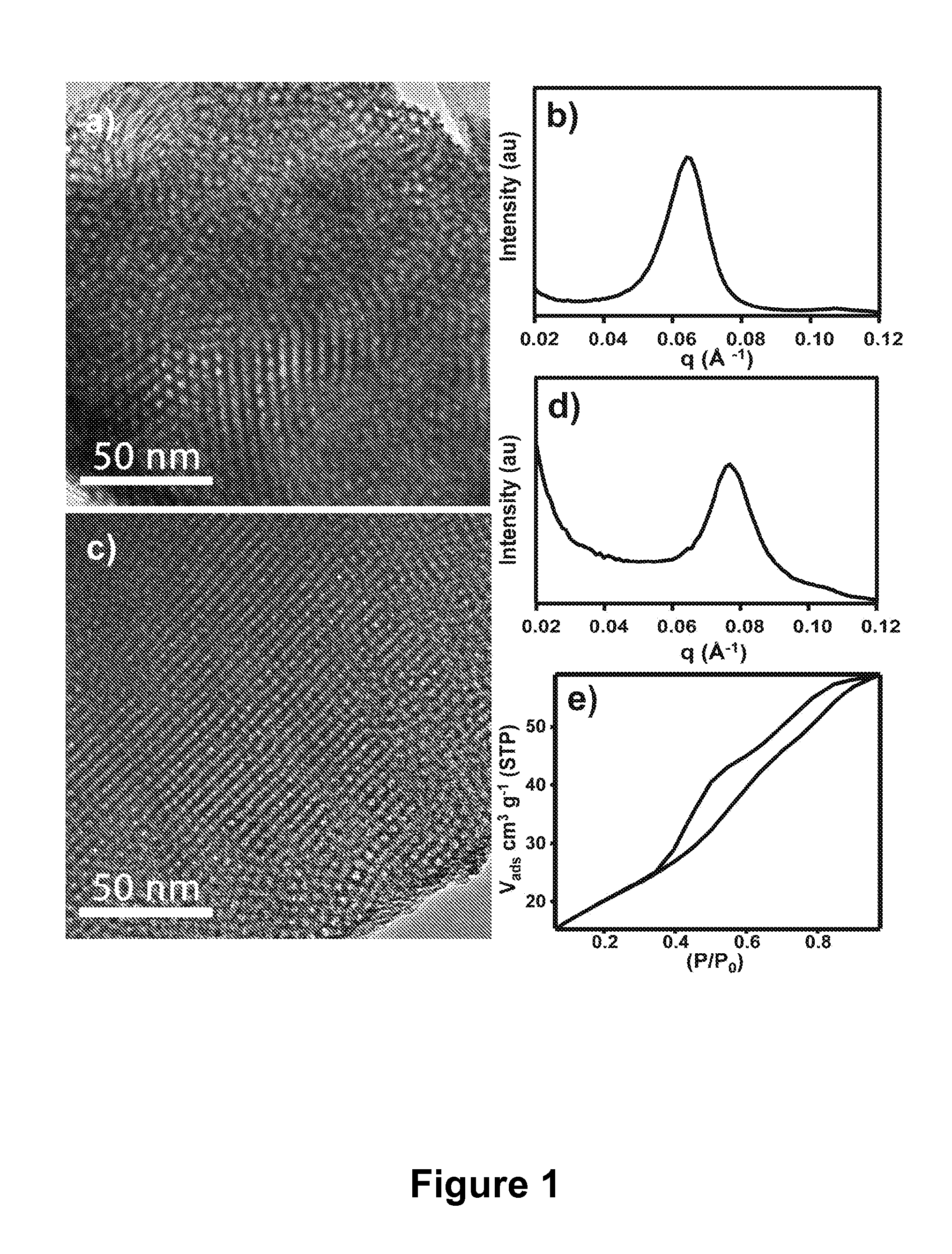
ActiveUS20100093102A1Strong specificityIncrease load capacityComponent separationPeptide preparation methodsPhosphoric acidMesoporous material
The present invention provides methods and materials for isolating, purifying, and / or enriching the concentration of compounds having one or more phosphate groups and / or derivatives thereof, including but not limited to phosphorylated peptides and / or phosphorylated proteins. In some aspects, the present invention provides nanostructured enrichment materials, such as metal oxide mesoporous materials, that selectively and reversibly bind with phosphorylated compounds with high specificity and are capable of controlled release of phosphorylated compounds bound to their active surfaces. Mesoporous materials of the present invention also provide enrichment materials having large active surface areas that provide for higher loading capacities for phosphorylated peptides and proteins relative to conventional affinity based methods. Nanostructured metal oxide mesoporous enrichment materials of the present invention are also compatible with implementation via a variety of separation platforms including flow through separation systems, elution based separation systems, column chromatography and affinity chromatography.
Owner:WISCONSIN ALUMNI RES FOUND
Applications of ZrO2 in the process for concentrating and purifying phosphorylated peptides
InactiveCN101284864ALarge specific surface areaHigh selectivityPeptide preparation methodsPhosphopeptidePhosphorylation site
The invention relates to the enrichment and purification of phosphopeptide in phosphoproteome, in particular to the application of ZrO2 in the enrichment and purification of phosphopeptide. ZrO2 nano material shows high specificity, selectivity, binding capacity and sensitivity at a phosphopeptide section; meanwhile, the affinity of the ZrO2 nano material to the specificity of phosphopeptide ensures that the material can be used in phosphoproteome during protein post-translational modification. Due to the specificity combination of ZrO2 with the phosphopeptide section in complex proteolysis sample, large-scale identification of phosphorylated protein and determination of phosphorylation sites can be realized through combining with MALDI-TOF MS and nano-LC MS / MS.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Systems and methods for the analysis of protein phosphorylation
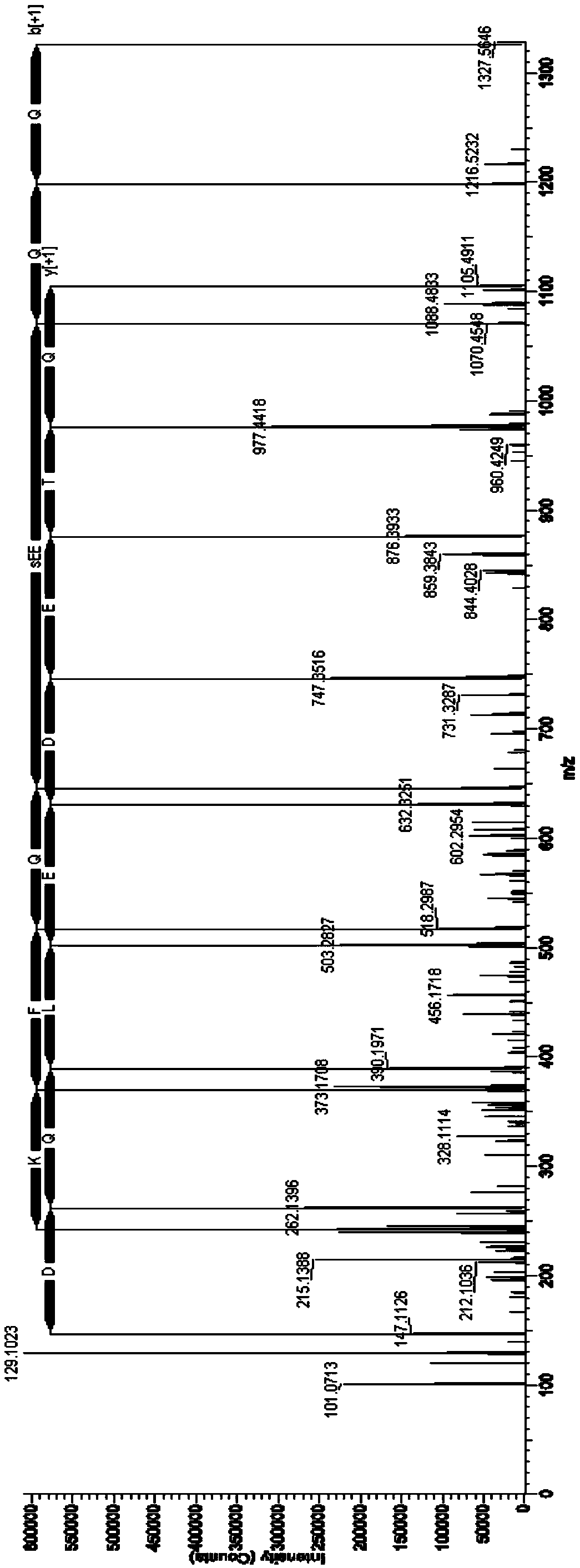
The present invention relates to a method of applying mass spectrometry to analyzing peptides or proteins, especially in the proteome setting. More particularly, the invention relates to a mass spectrometry-based method for detection of protein / peptide phosphorylation wherein the side chains of glutamic acid and / or aspartic acid residues of said peptides or proteins are chemically modified as to improve the selectivity / efficiency of identification of the phosphorylated protein / peptide.
Owner:PROTANA +1
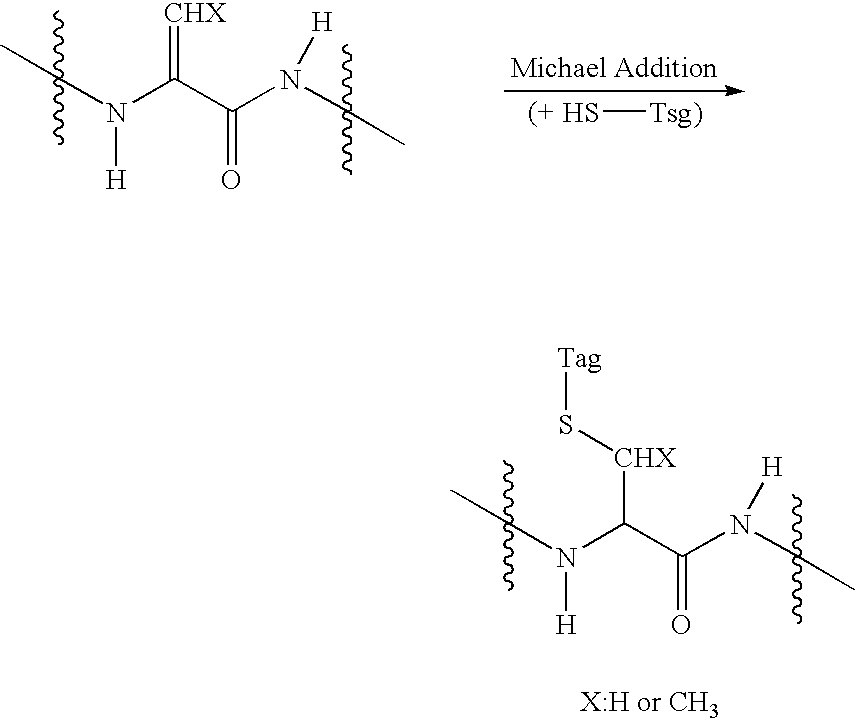
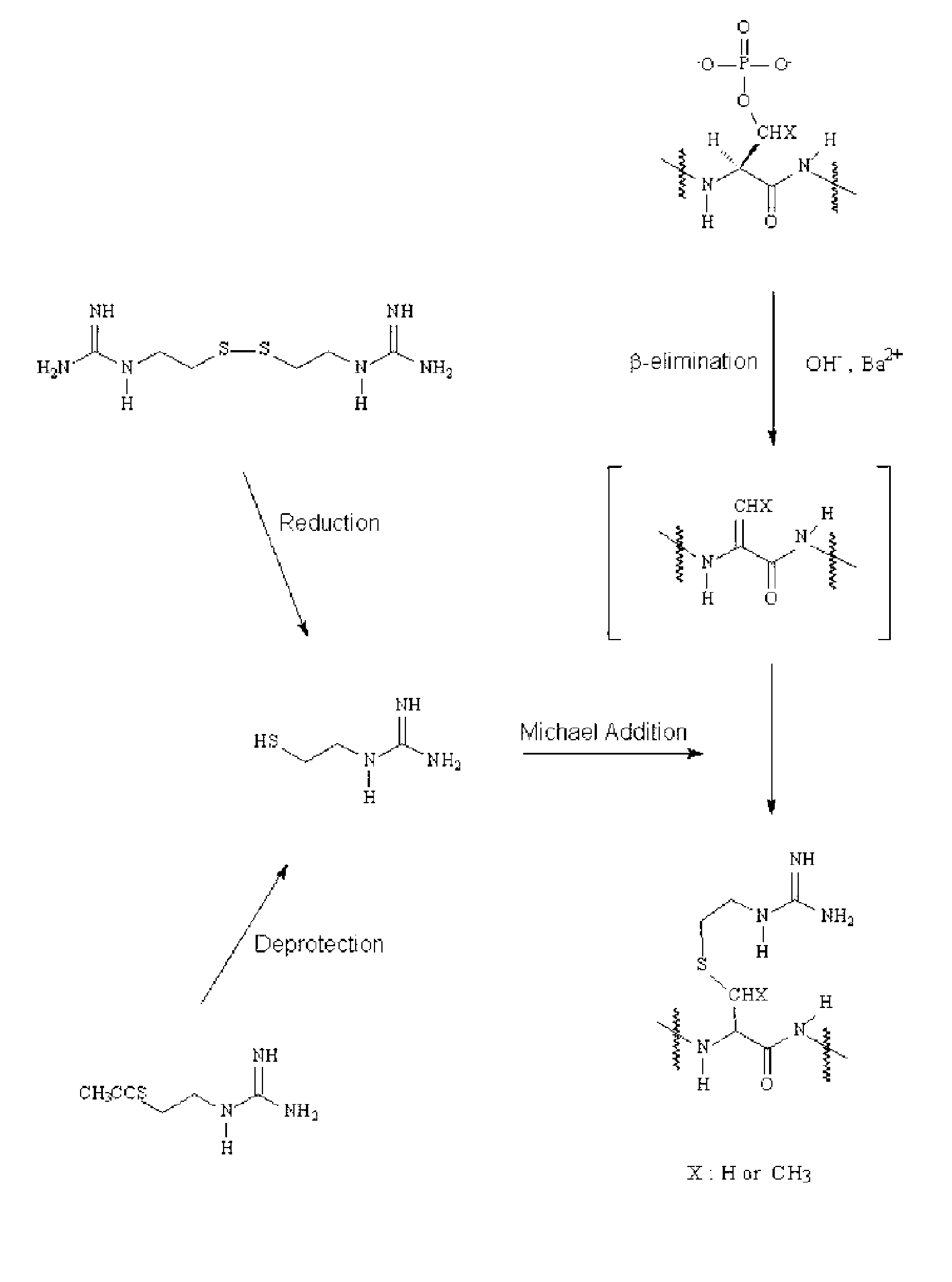
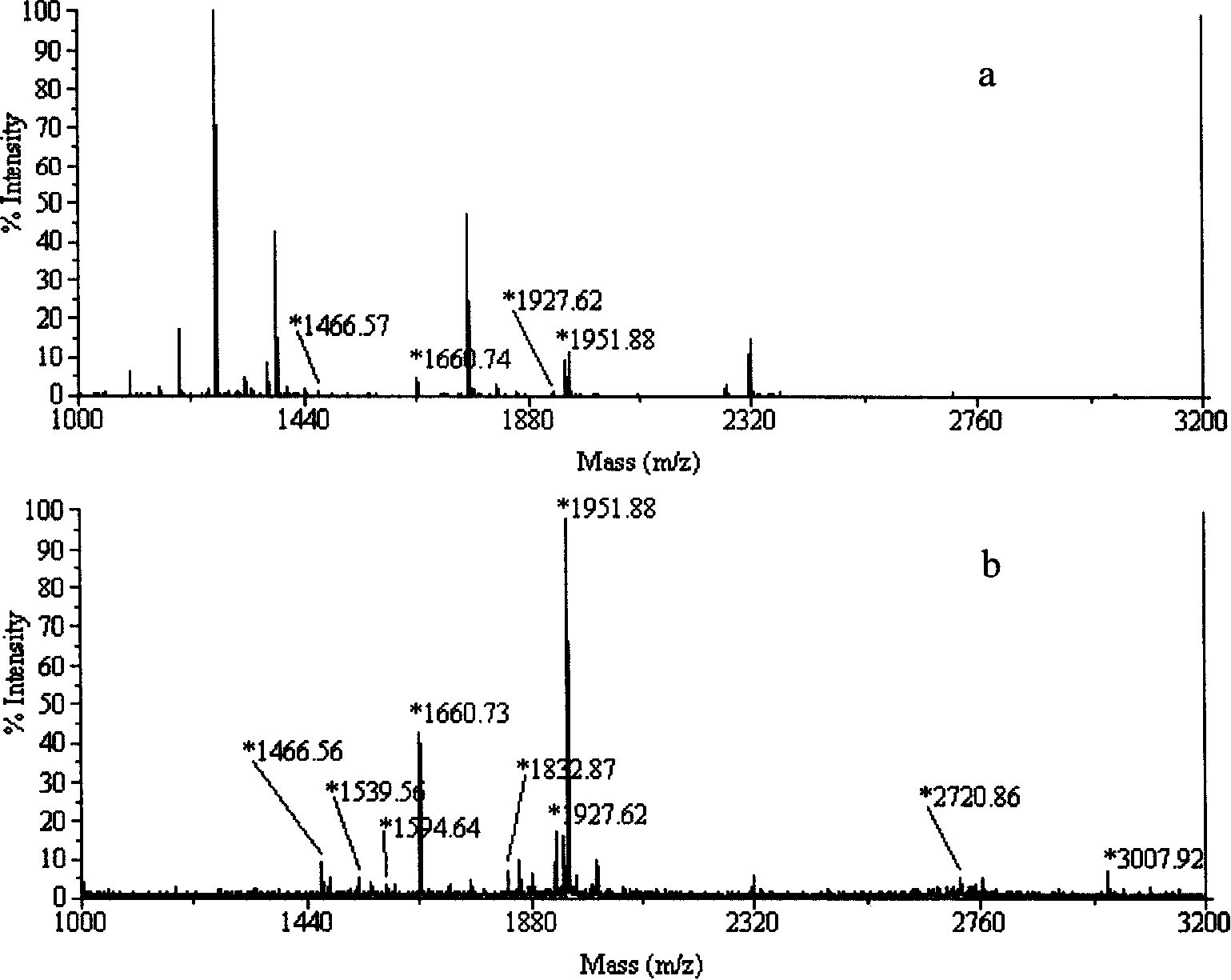
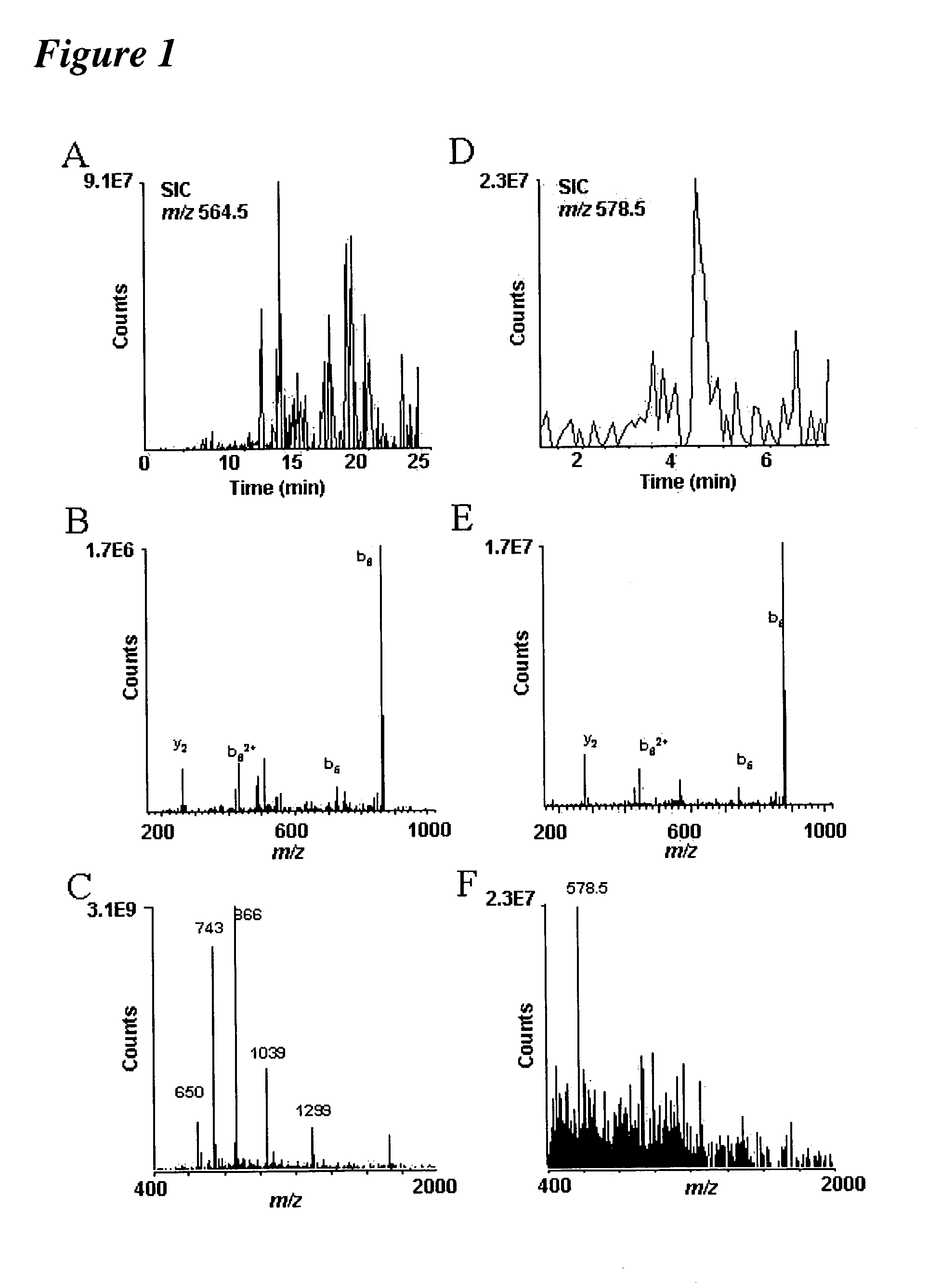
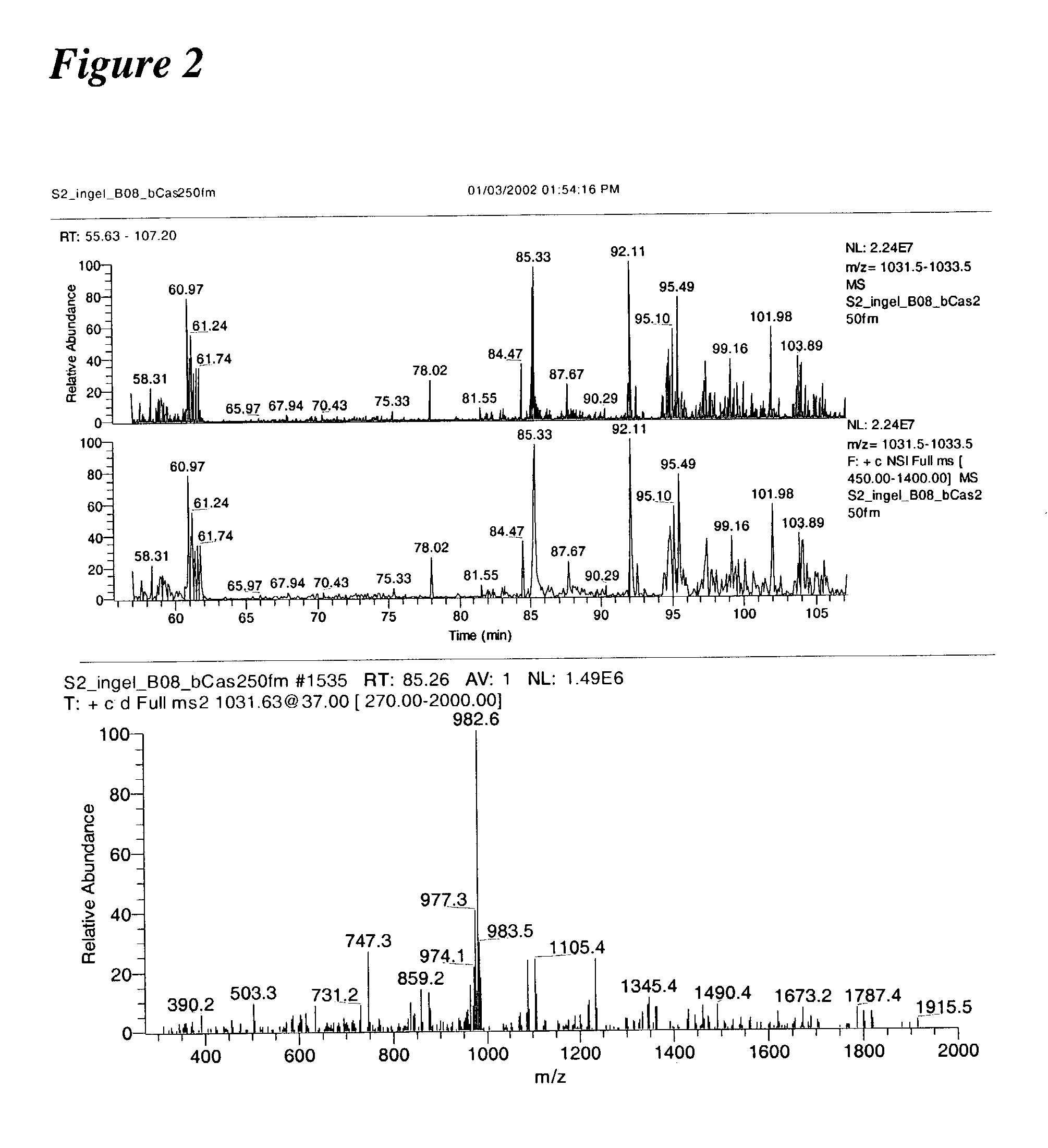
In-gel tagging and in-gel digestion for phosphoproteins analysis and phosphorylation site identification
The present invention relates to a method for phosphorylation site-specific labeling of phosphoproteome with a site-specific tagging reagent and analyzing of the resulting labeled one, more especially, a method for in-situ tagging of phosphorylation sites of phosphoproteins retained in polymeric gel with a nucleophilic tagging reagent. It also relates a method for generating new proteolytic cleavable sites at formerly phosphorylation sites by a proper choice of a nucleophilic tagging reagent. It also relates to a method for phosphopeptides analysis and phosphorylation site identification by in-gel digestion of the previously in-gel tagged proteins and subsequent mass analysis of the resulting peptides. The invention provides in-gel chemical tagging method for phosphoaminoacid residue of phosphoproteins retained in polymeric gel matrix. Phosphoprotein can be immobilized into gel matrix by a variety of methods such as gel electrophoresis. The immobilized phosphoproteins are retained in gel matrix during tagging reaction to phosphorylated aminoacid residue of phosphoproteins, and the resulting tagged proteins are also retained in gel matrix till following purification steps like washing of the tagging reagents are accomplished. The tagged proteins is digested by protease, and the resulting digested peptides is released from gel into solution and applied for peptide mass analysis.
Owner:KOREA BASIC SCI INST
Method for quantifying phosphokinase activity on proteins
InactiveUS20090104628A1Microbiological testing/measurementBiological material analysisProtein targetTyrosine
The invention involves a method for measuring phosphorylation of proteins at specific sites and, as such, is an indicator of the protein kinase activity of enzymes capable of phosphorylating those sites. The method involves the in vitro or in vivo phosphorylation of a target protein at a specific serine, threonine or tyrosine residue, subjecting that protein (non-phosphorylated) to reaction mixture containing all reagents, including phosphokinase which allow the creation of a phosphorylated form of protein. The phosphorylated protein is measured by contacting it with an antibody specific for the phosphorylation site(s). The invention includes antibodies useful in practicing the methods of the invention. The invention particularly relates to all proteins modified by phosphorylation and dephosphorylation as illustrated by Tau, Rb and EGFR proteins and antibodies specific for the site of phosphorylation of the Tau, Rb or EGFR proteins.
Owner:LIFE TECH CORP
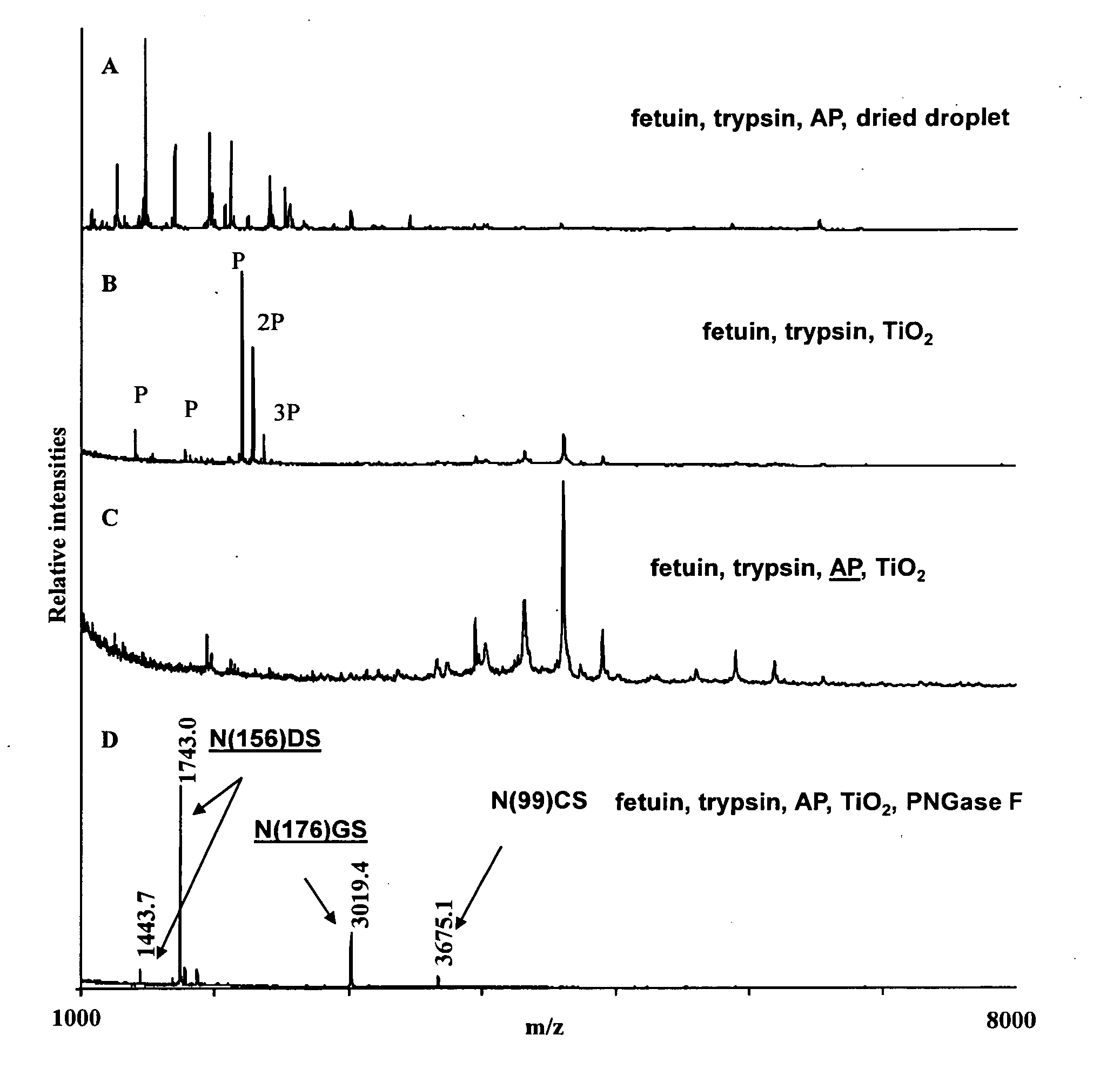
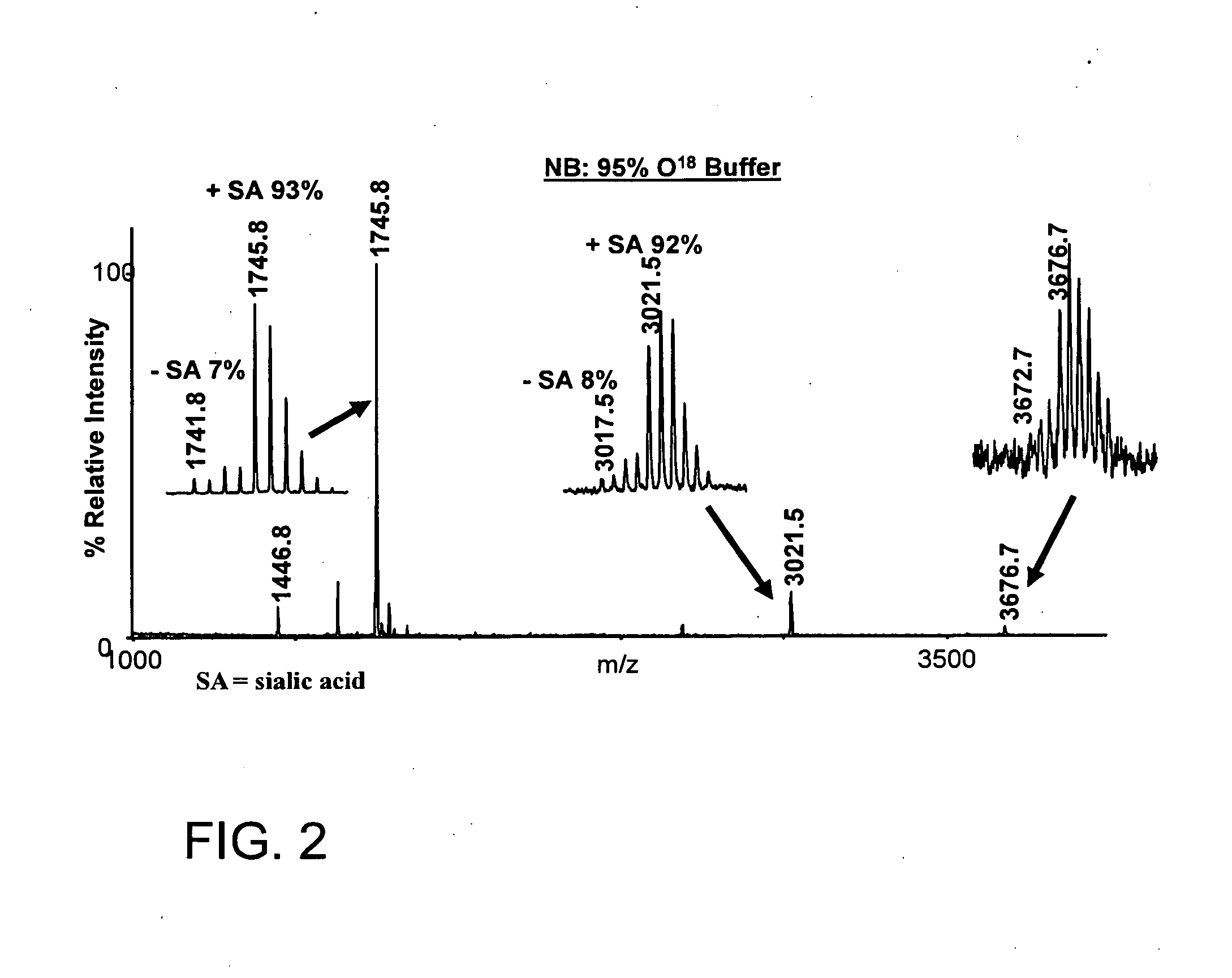
Methods for isolation and analysis of sialylated and phosphorylated peptides
ActiveUS20090029343A1Easy to identifyEasy to comparePeptide/protein ingredientsOvalbuminPhosphorylationCarboxylic acid
Changes in sialylation of cell surface or plasma proteins are often associated with various cancers and other disease conditions Provided are methods of detecting biomarkers of conditions associated with a change of sialylation status. Sialylated peptides are first isolated from biological and other samples by loading onto titanium dioxide (TiO2) or zirconium dioxide (ZrO2) stationary phase under acidic conditions in a solution comprising at least 20% organic phase and at least about 6.5 mM of substituted aromatic carboxylic acid, or, alternatively, at least about 1 mM short chain, non-aromatic, hydroxylated carboxylic acid. Sialic acid containing proteins can then be eluted from loaded stationary phase material by exposure to an alkaline solution having pH of 9.0 or greater, preferably at least 10.5. Sialylated peptides isolated by the methods provided can then be analysed by mass spectrometry to identify patterns of sialylation across a sialiome (the entire complement of sialic acid containing peptides in a biological sample) and / or to identify proteins in a sample that are sialylated or that show changes in sialylation status between two or more different samples. In preferred embodiments, sialylated peptides from control and patient samples can be differentially isotopically labelled and compared in a single mass sprectrometry experiment. Also provided are specific biomarkers of bladder cancer. The methods for isolating and analysing sialylated proteins can also by applied to phosphorylated proteins.
Owner:SYDDANSK UNIV
Expression vectors for producing modified proteins
InactiveUS6514753B1Phosphorylation reaction isExtended shelf lifeBacteriaPeptide/protein ingredientsEscherichia coliMicroorganism
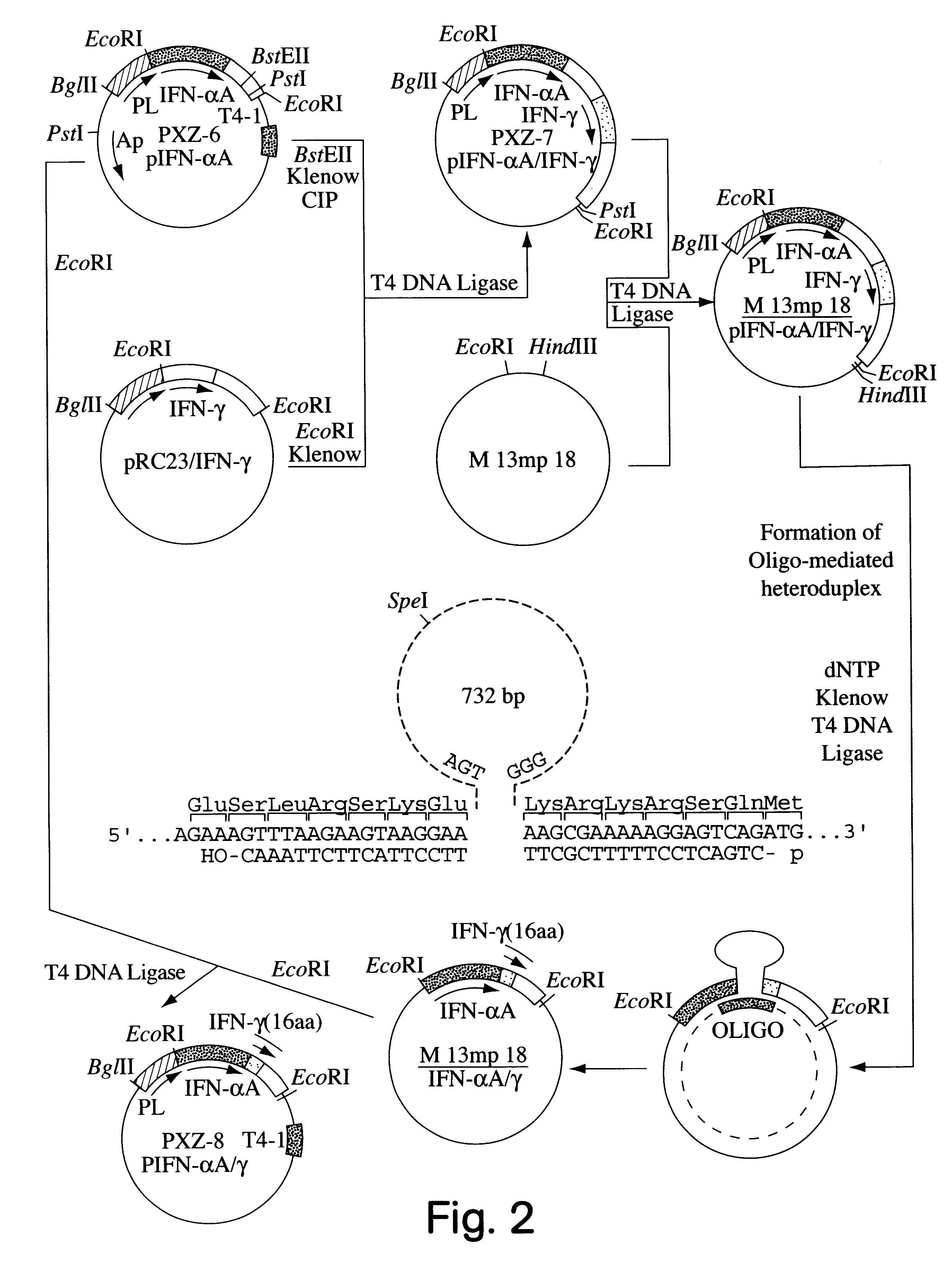
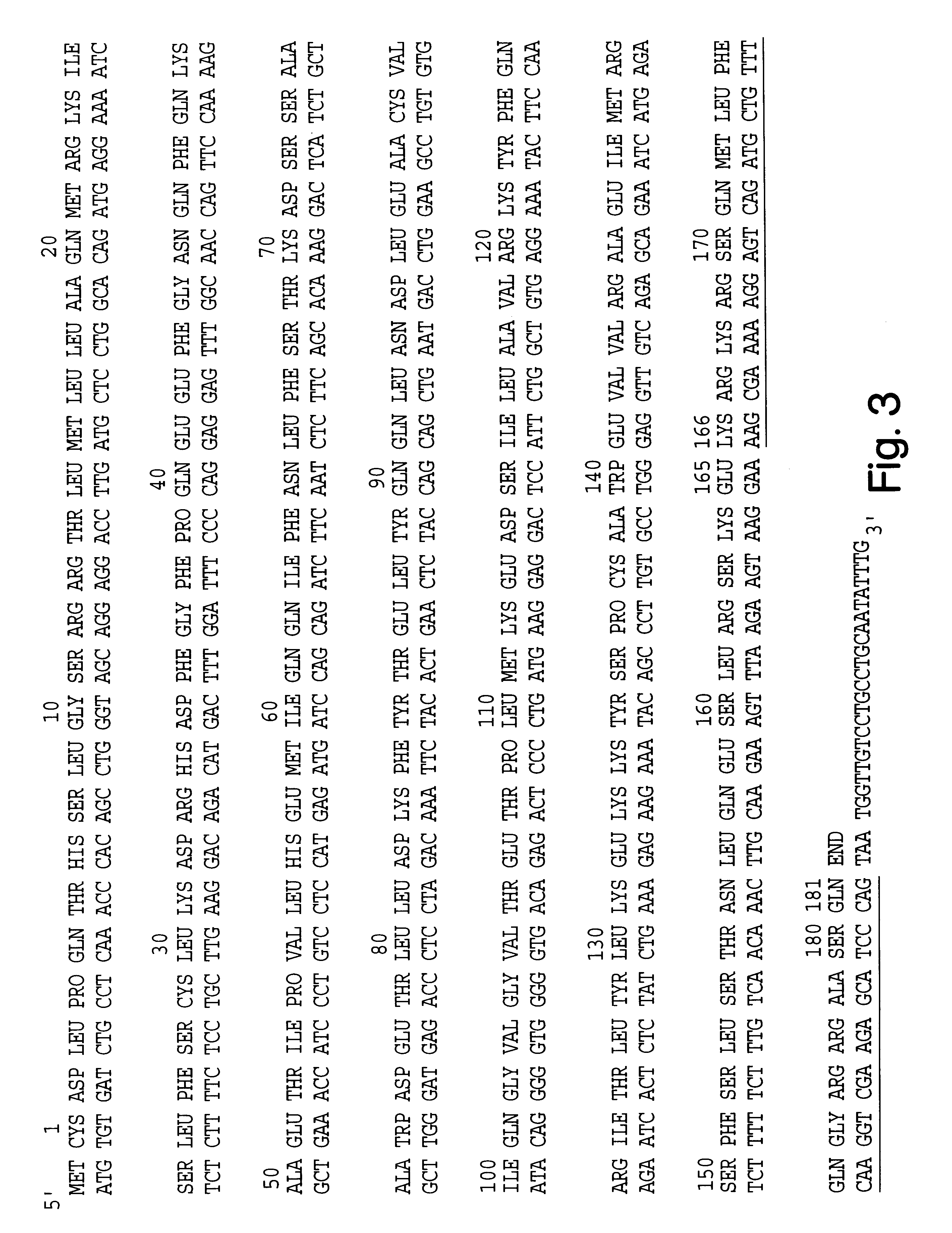
Modified proteins, modified interferons alpha's and beta's, phosphorylated modified proteins and DNA sequences encoding the above, applications and uses thereof. Modified phosphorylated Hu-IFN-alpha-like proteins are provided which carry an identifiable label such as a radio-label. Corresponding phosphorylatable Hu-IFN-alpha-like proteins which contain a putative phosphorylation site. DNA sequences which encode a Hu-IFN-alpha-like protein and contain a sequence encoding a putative phosphorylatable site. Appropriate expression vectors are used to transform compatible host cells of various microorganisms, such as E. coli. Numerous uses for the phosphorylated proteins are disclosed.
Owner:PESTKA BIOMEDICAL LAB
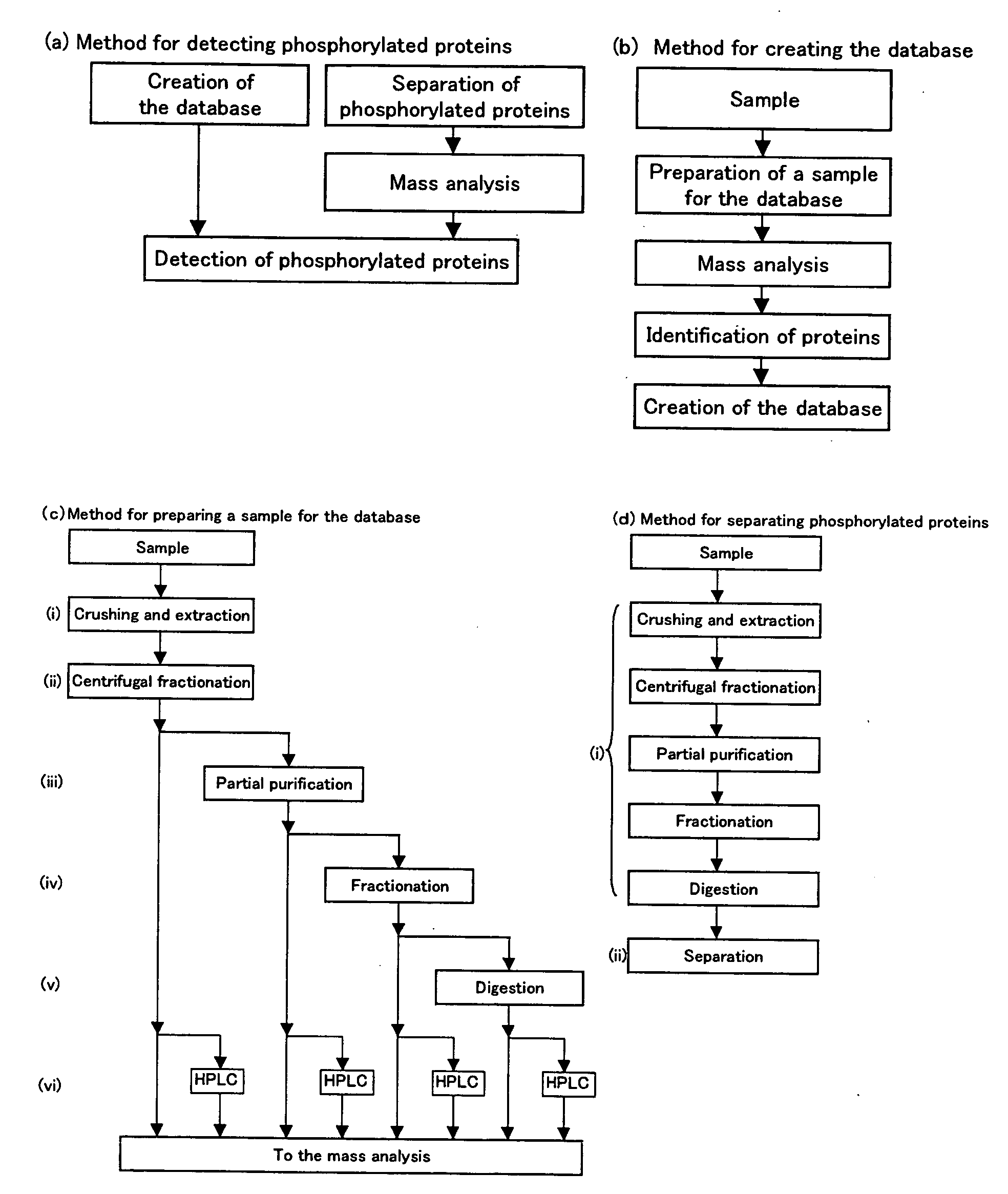
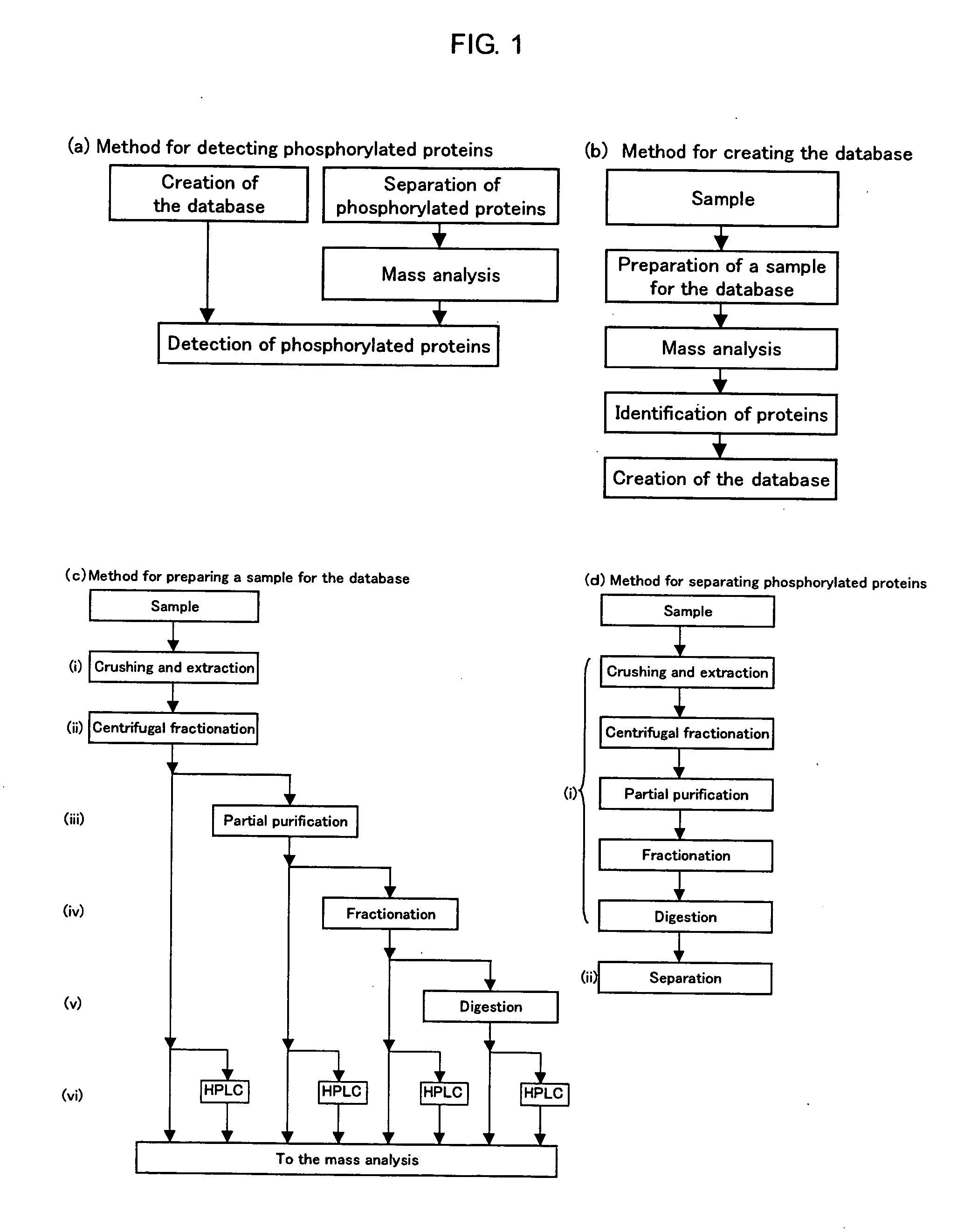
Method of Proteome Analysis for Phosphorylated Protein
InactiveUS20080221802A1Improve accuracyShort timeParticle separator tubesComponent separationAcetonitrileProteome
A method for detecting plural types of phosphorylated proteins in a sample, wherein a database consisting of data regarding plural types of proteins in the sample is used; and a method for purifying phosphorylated proteins using an immobilized metal carrier or a titania carrier, wherein a solution containing acetonitrile in a range of 40% (v / v) or greater but 60% (v / v) or less is used.
Owner:EISIA R&D MANAGEMENT CO LTD
Method for quantitatively analyzing tyrosine-phosphorylated proteins
InactiveCN108693348ALow detection sensitivityStrong specificityMaterial analysisReference samplePre enrichment
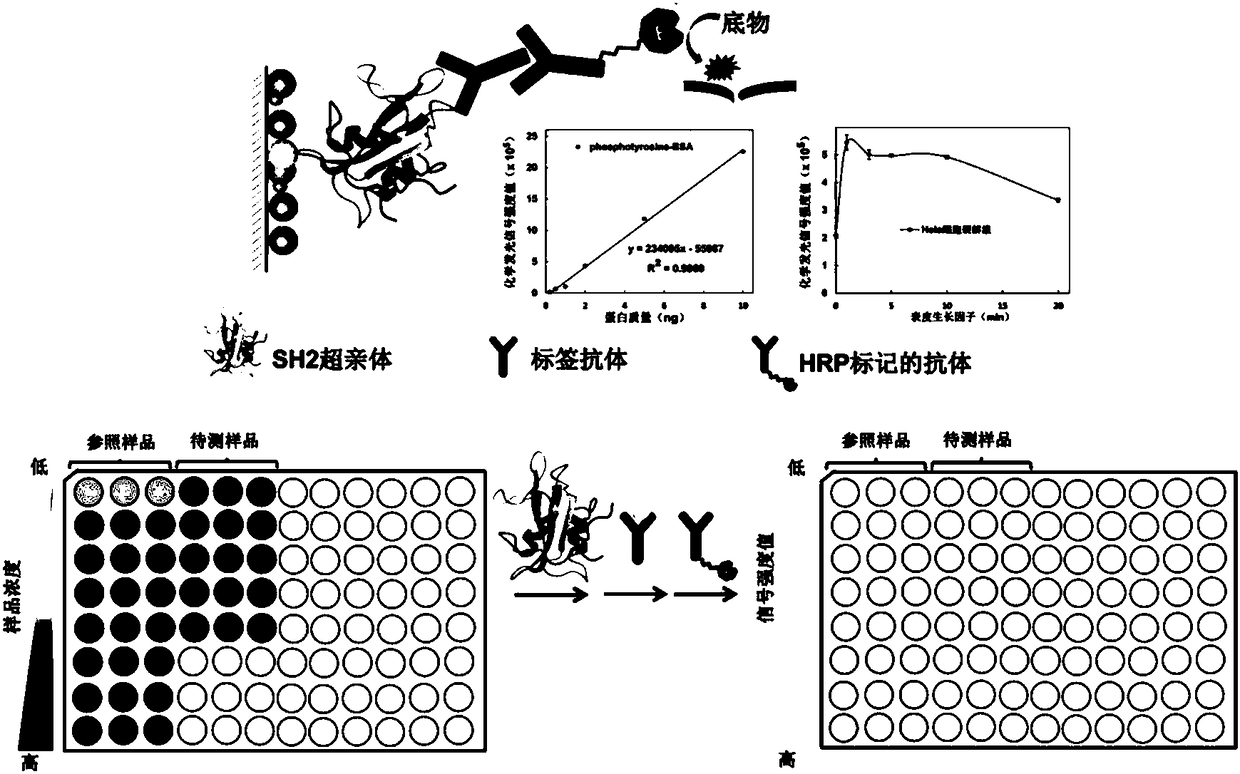
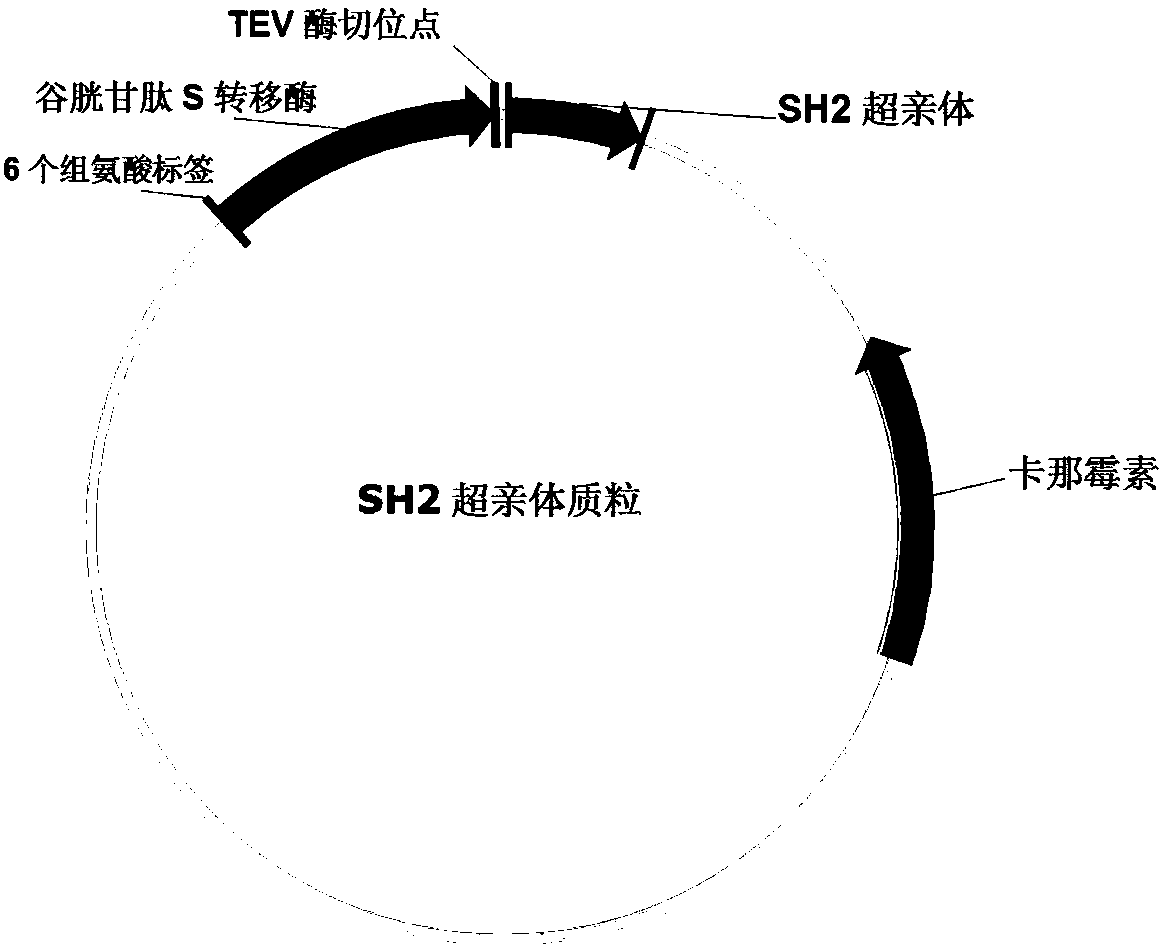
The invention relates to a method for quantitatively analyzing tyrosine-phosphorylated proteins. The method is characterized in that the dynamic change curve of the tyrosine phosphorylation level of asample to be tested is obtained based on an ELISA technology with a modified SH2 over parent as a first antibody, a standard curve is obtained with tyrosine-phosphorylated standard proteins as a reference sample in the detection process, and the quantitative analysis of the tyrosine phosphorylation level of the sample to be tested is realized with the standard curve as a reference. The method hasthe advantages of low cost, no sample pre-enrichment, good specificity and low detection limit. The ELISA technology is carried out in a well plate, and an apparatus is simple. The SH2 over parent adopted in the invention can be used to obtain a lot of proteins with a stable structure through bacterium expression and purification, so the experimental cost is greatly saved, the background is clear, and reconstruction is easy. The method has the same high affinity and high specificity to the tyrosine-phosphorylated proteins as antibody, has a broad spectrum, and can successfully realize the quantitative analysis of the tyrosine phosphorylation level of multiple complex samples.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method of separating phosphorylated peptide or phosphorylated protein
InactiveUS20100012832A1Improve efficiencyParticle separator tubesPeptide/protein ingredientsADAMTS ProteinsPhosphoric acid
According to the present invention, phosphorylated peptides and / or phosphorylated proteins are specifically separated. A sample containing a phosphorylated peptide and / or a phosphorylated protein is supplied to a separation unit filled with a metal oxide in the presence of an aliphatic hydroxycarboxylic acid. Upon separation of a phosphorylated peptide and / or a phosphorylated peptide with the use of a separation unit filled with a metal oxide, adsorption of carboxylic acid to an acidic peptide can be prevented in the presence of aliphatic hydroxycarboxylic acid. In addition, aliphatic hydroxycarboxylic acid does not inhibit adsorption of a phosphorylated peptide and a phosphoric acid group in the phosphorylated peptide to a metal oxide.
Owner:KEIO UNIV
Preparation method of flexible metallic oxide nanofiber phosphorylated peptide enrichment material
InactiveCN109095894AImprove continuityHigh content of target metal oxidesInorganic material artificial filamentsNanofiberRoom temperature
The invention discloses a preparation method of a flexible metallic oxide nanofiber phosphorylated peptide enrichment material. The preparation method of the flexible metallic oxide nanofiber phosphorylated peptide enrichment material includes the steps that 1, metal salt is added into corresponding solvent, stirring is conducted to generate metal ions, then, a chelating agent is added, stirring is conducted again, and a precursor solution is obtained, wherein the ratio of the metal salt to the solvent is 1 g:(10-80) ml, and the molar ratio of the metal salt to the chelating agent is 1:(0.01-0.4); 2, the obtained precursor solution is subjected to electrostatic spinning to obtain a precursor nanofiber membrane; 3, the precursor nanofiber membrane is calcined in air, the calcining temperature gradually increases to 500-1200 DEG C from room temperature, calcining is kept for 30-120 min at the highest calcining temperature, and then the flexible metallic oxide nanofiber membrane is obtained. The preparation method of the flexible metallic oxide nanofiber phosphorylated peptide enrichment material is simple in process, and phosphorylated protein and phosphorylated peptide fragments canbe effectively enriched and purified efficiently and repeatedly.
Owner:XI'AN POLYTECHNIC UNIVERSITY
Phosphopeptide enriching material and preparation method and application thereof
InactiveCN106749884AHigh selectivityImprove throughputPeptide preparation methodsAtom-transfer radical-polymerizationPhosphopeptide
The invention relates to the field of material analysis chemistry and organic chemistry. The invention provides a phosphopeptide enriching material and a preparation method and application thereof. The phosphopeptide enriching material comprises a substrate and a double-ingredient copolymer layer formed on the surface of the substrate, wherein the thickness of the double-ingredient copolymer layer is 10 to 80nm. The surface initiation-atom transfer free radical polyreaction mechanism is utilized; the double-ingredient copolymers are grafted on the surface of the substrate. The material and column solid phase extraction mode or dispersion solid phase extraction mode are organically combined; the enriching with high selectivity, high repetitiveness and high flux of phosphopeptide in a complicated mixture can be realized, so that the selective enriching of the multi-phosphopeptide and mono-phosphopeptide can be realized; the phosphorylated protein identification number can be obviously improved. Therefore the phosphopeptide enriching material is hopeful to be widely applied to the aspects of phosphopeptide enriching, further large-scale separation of phosphorylated protein and the like.
Owner:WUHAN UNIV OF TECH +1
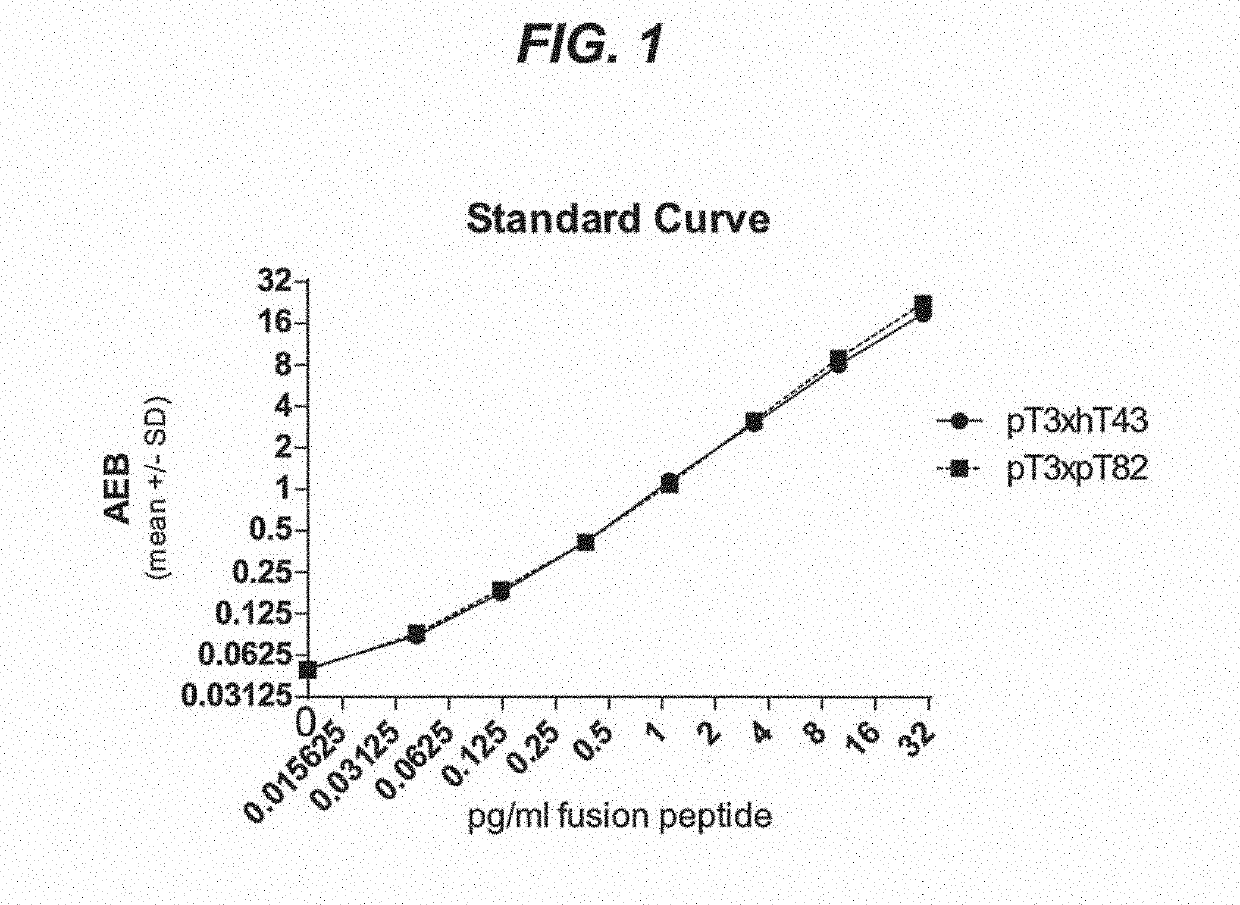
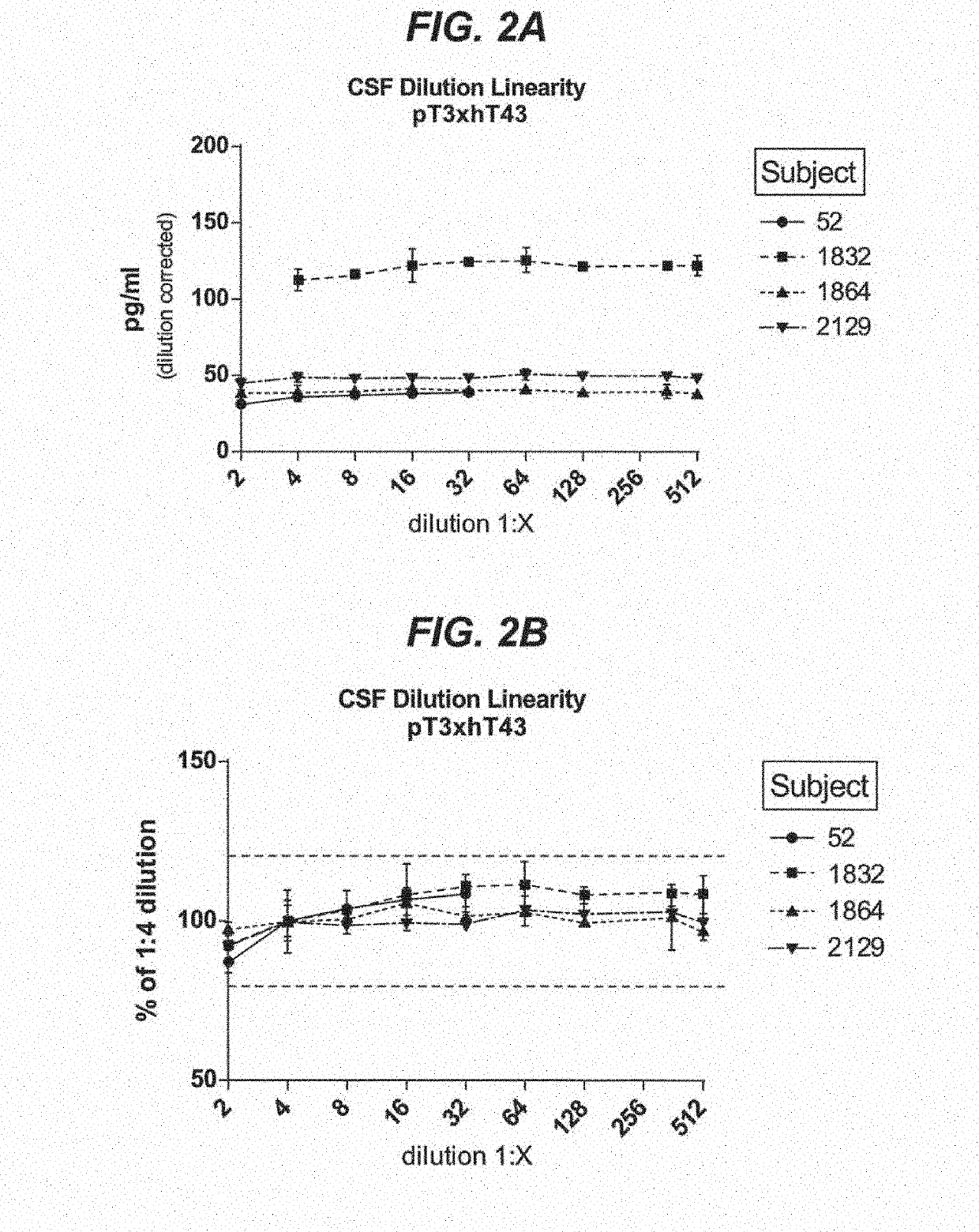
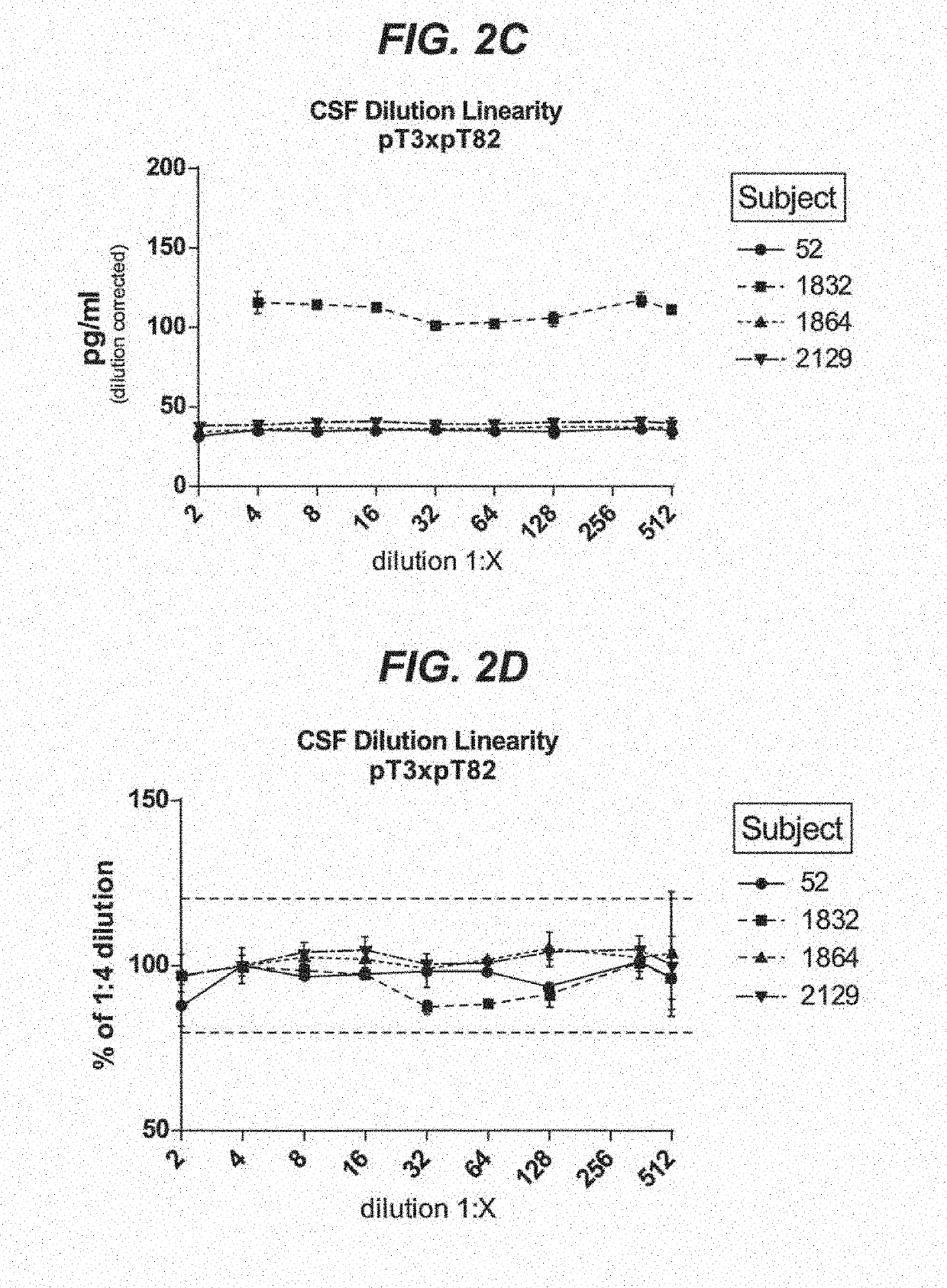
Assays to detect neurodegeneration
ActiveUS20190271710A1Highly sensitive, precise, accurateSamplingComponent separationAntiendomysial antibodiesAssay
Methods of measuring the amount of singly- or multiply-phosphorylated p217+ tau protein in a sample are provided. Methods of detecting or diagnosing tauopathies, methods of determining the effectiveness of a treatment of a tauopathy, and methods of determining whether a subject is suitable for anti-p217+ tau antibody therapy are also provided. Also described are antibodies for use in the methods and kits comprising the antibodies.
Owner:JANSSEN PHARMA NV
Preparation of organic phosphonic acid functionalized magnetic nano material and application in protein enrichment
InactiveCN101923934ARealize the combinationImprove bindingComponent separationOrganic/organic-metallic materials magnetismSynthesis methodsPhosphorylation
The invention provides an R-CH2-PO3-M modified magnetic nanoparticle as a phosphorylation protein enrichment material, as well as a preparation method and application thereof in phosphorylation protein enrichment. The purpose of combining the functions of magnetic nanoparticles and phosphonic acid groups can be achieved through modifying carboxyl groups for organic phosphonic acid gene R. The synthesis method of the magnetic nano material with functionalized organic phosphonic acid is simple, rapid and green. The material has favorable enrichment effect on phosphorylation proteins, and an MALDI-TOF MS (Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry) can be used for detecting a phosphorylation peptide segment in 10-8M of bovine serum beta-casein digestive juice. The results show that a type of favorable phosphorylation protein enrichment materials can be constructed through combining the magnetic nanoparticles with the organic phosphonic acid.
Owner:NANJING UNIV
Tyrosine phosphoproteomics analysis method of tyrosine phosphopeptide
InactiveCN108414606AStrong specificityEasy to operatePreparing sample for investigationOmicsTrifluoroacetic acidElution
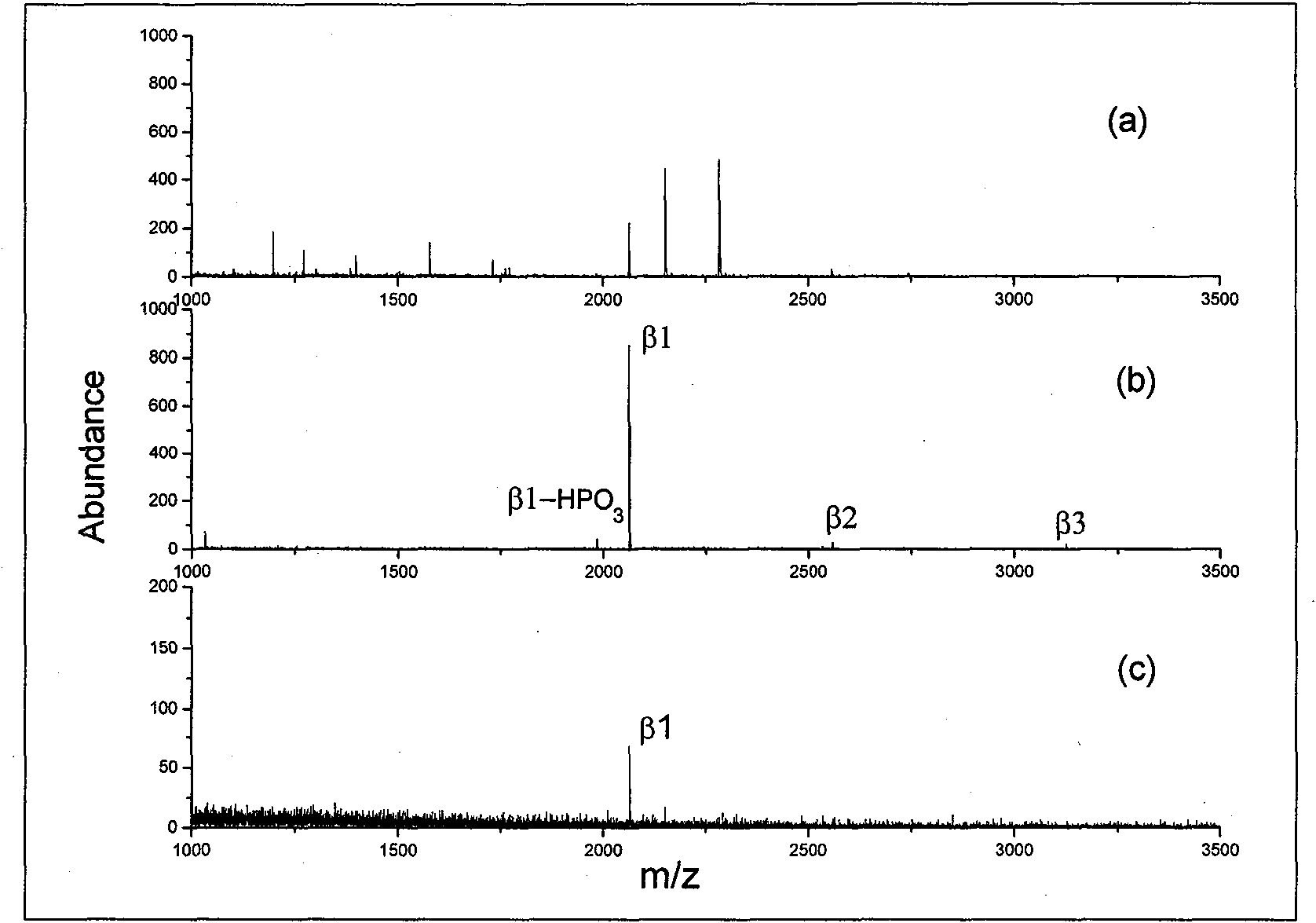
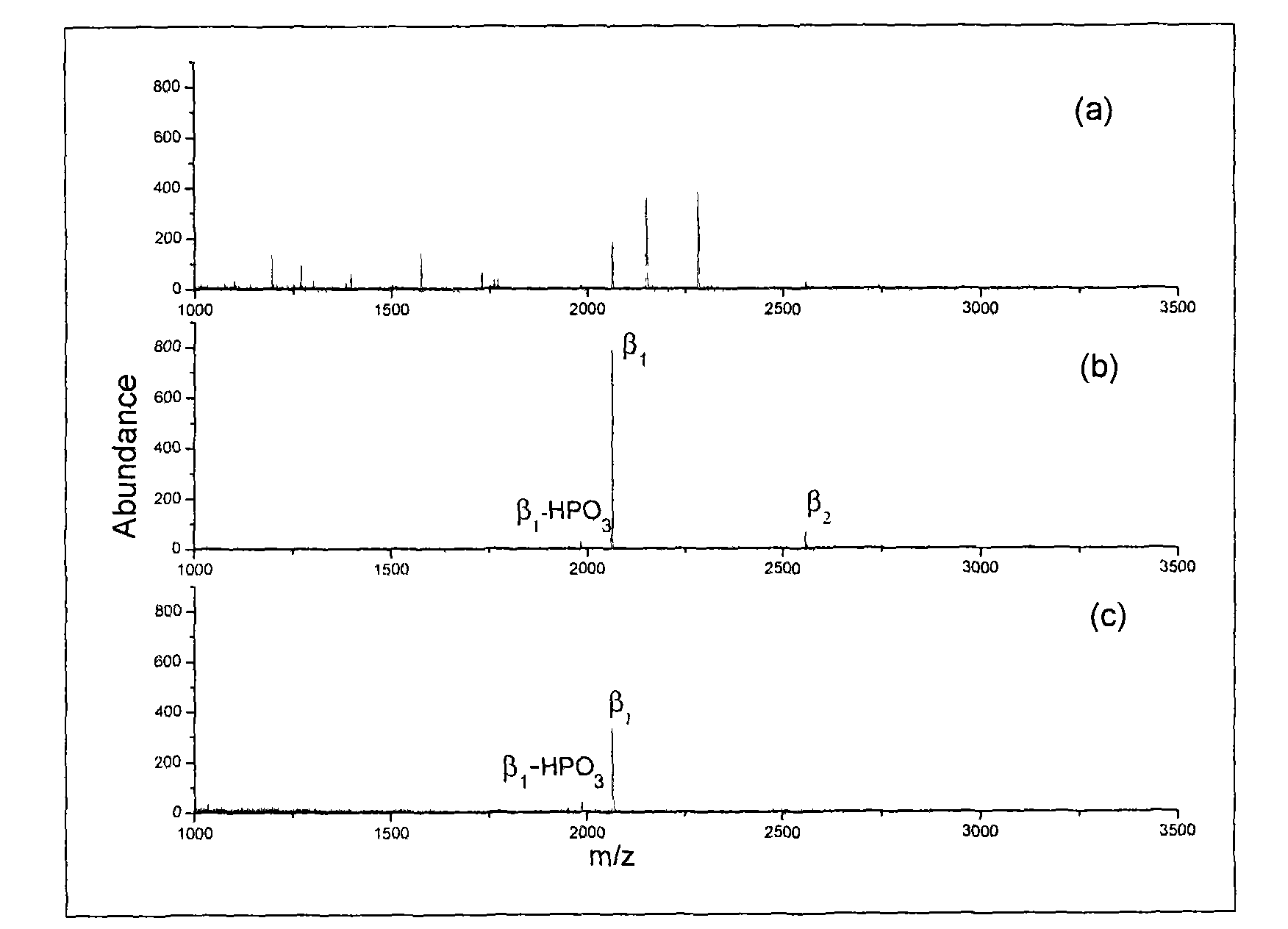
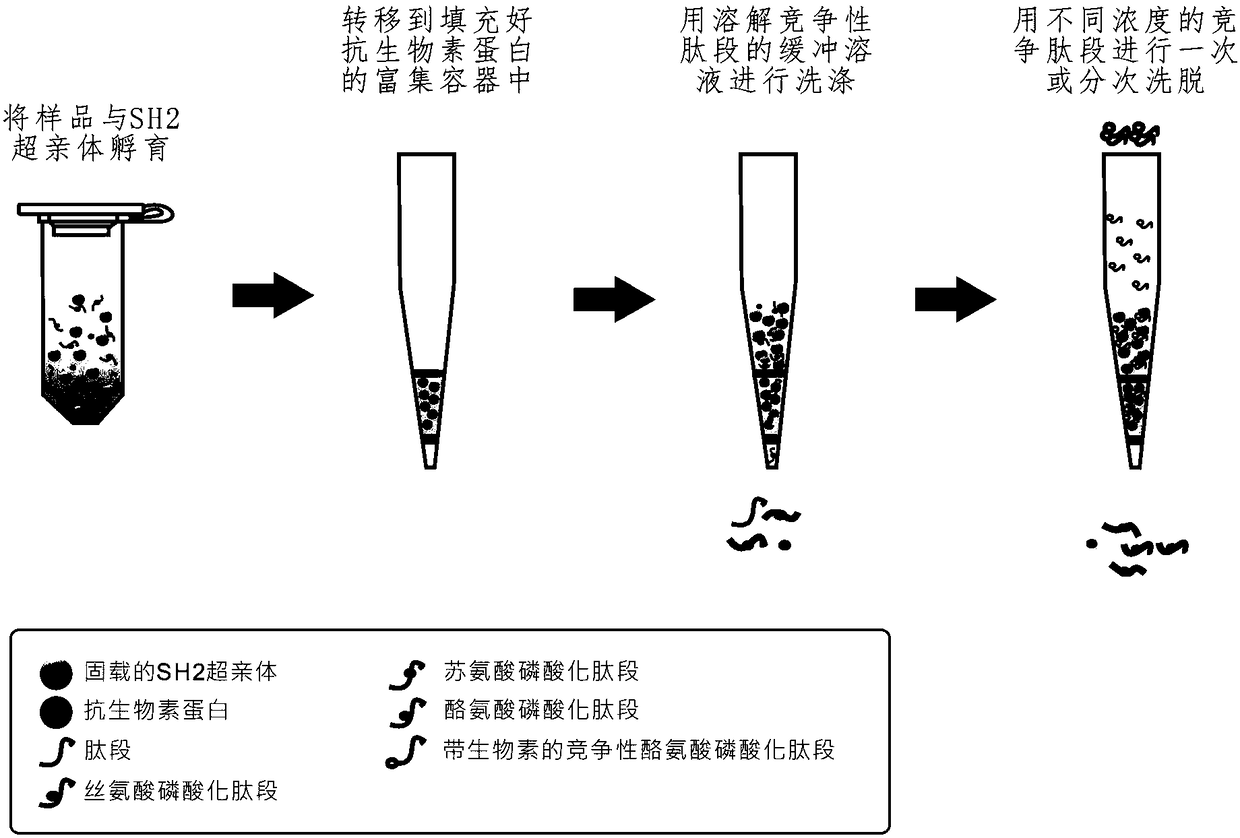
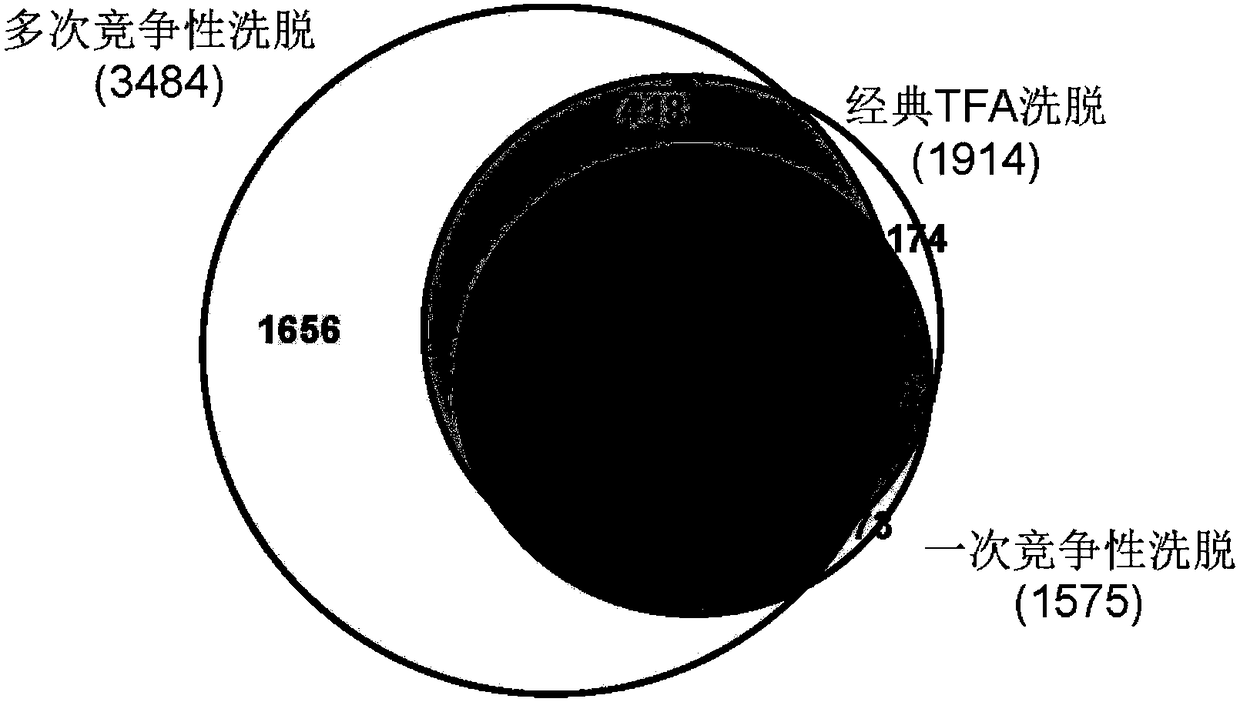
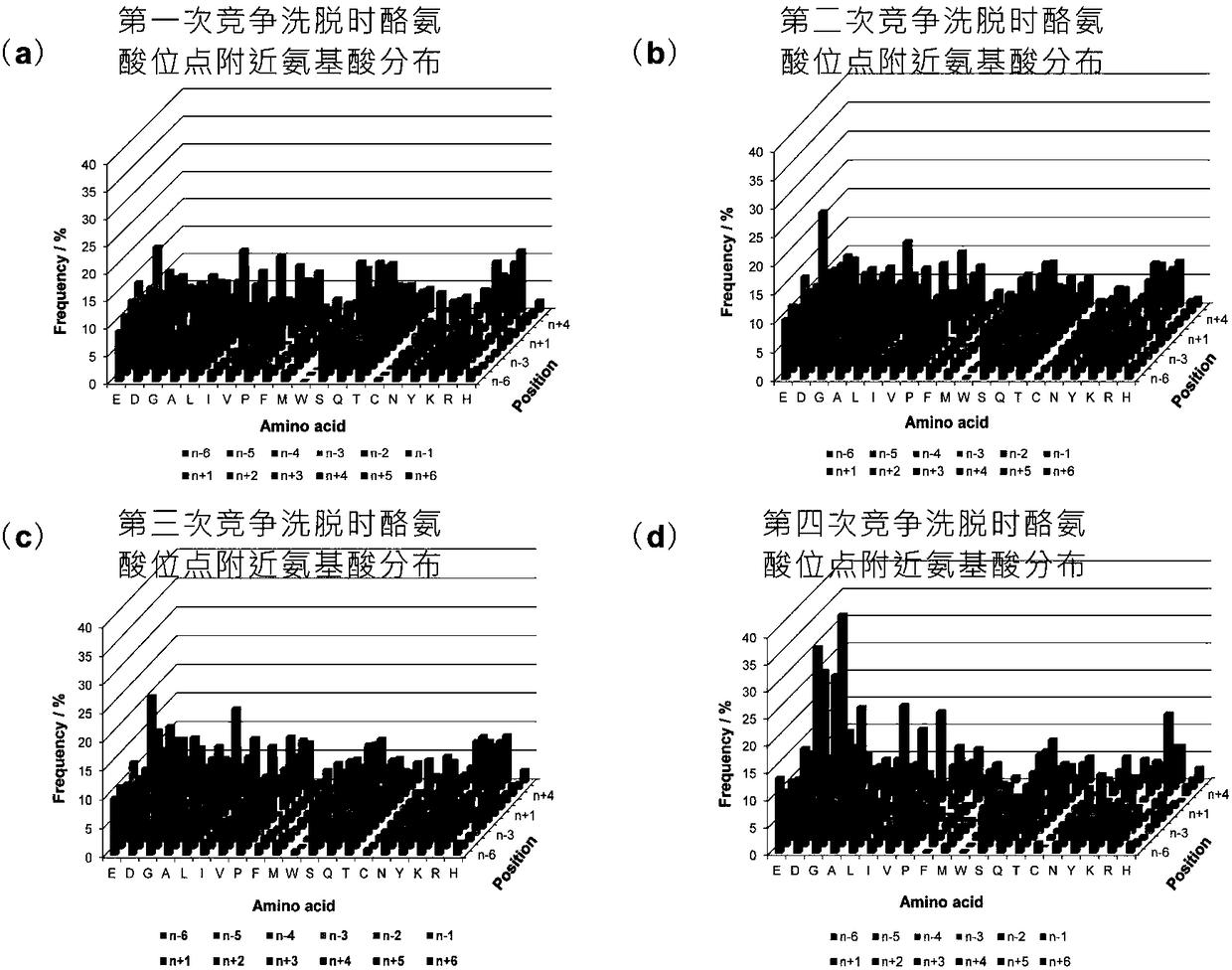
The present invention relates to a method for deep analysis of tyrosine phosphoproteomics based on competitive elution of affinity tyrosine phosphopeptide on a superbinder SH2. Specifically in the elution process of tyrosine phosphopeptide enrichment, a biotin-containing tyrosine phosphopeptide capable of strongly interacting with a superbinder SH2 is used as a competitive reagent to replace the traditional trifluoroacetic acid, the tyrosine phosphopeptide acting with the superbinder SH2 is sequentially eluted according to the intensity of the interaction, and the competitive reagent being notbound to the superbinder SH2 is bound by corresponding avidin so as not to affect the subsequent identification and analysis of the tyrosine phosphopeptide sample. According to the present invention,the method has advantages of rapidness, sensitivity, low cost and the like, can easily achieve the deep analysis of tyrosine phosphoproteomics, and can further provide the information of the interaction between the tyrosine phosphopeptide and the superbinder SH2.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
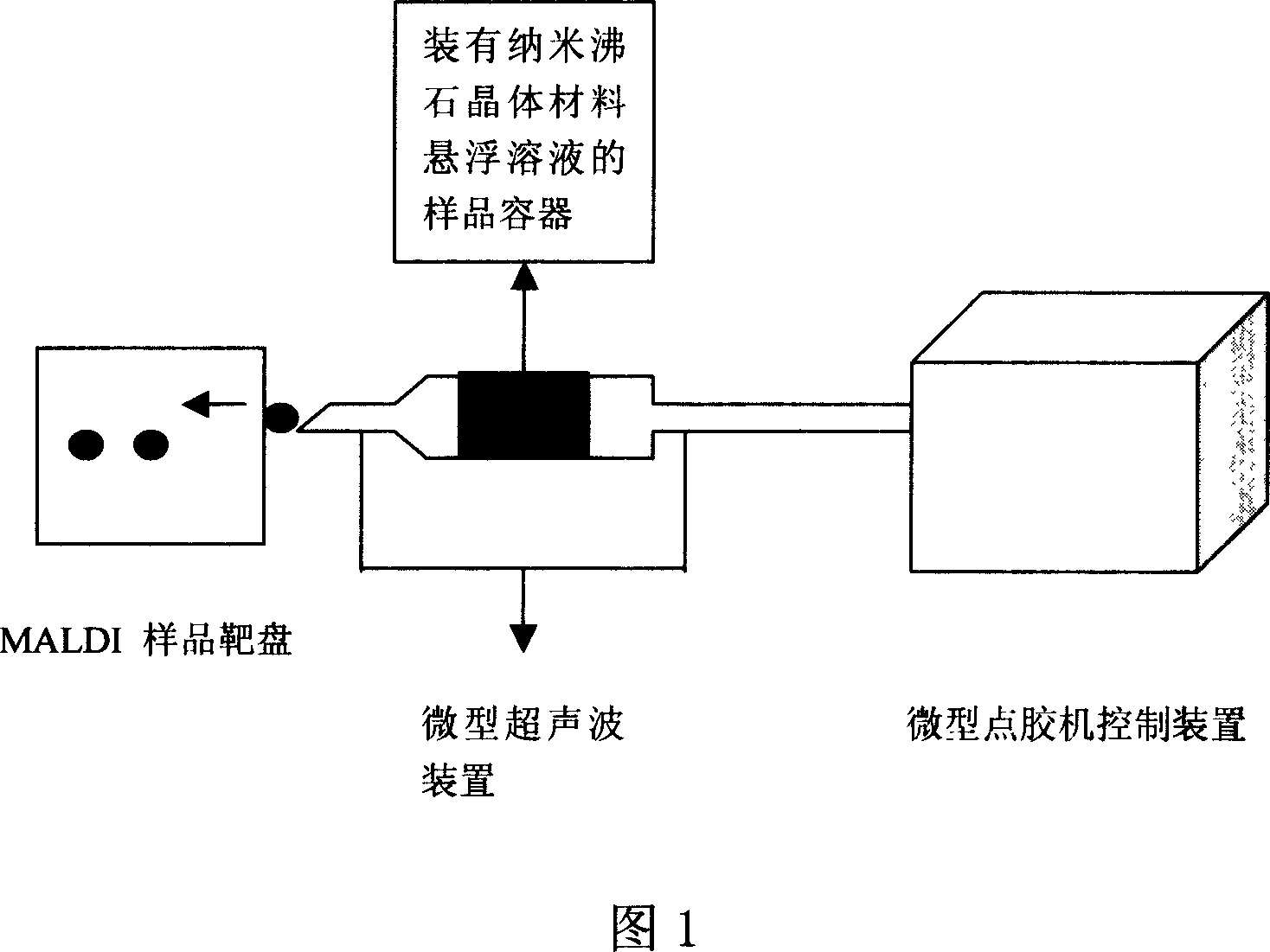
Method for preparing functional target disk of mass spectrum sample de-absorbed by ground substance assistant laser
InactiveCN101004408AOvercome technical difficultiesCompletely innovativeIon-exchange process apparatusComponent separationCooking & bakingEthyl acetate
A method for preparing mass spectrum sample function target plate of base material auxiliary laser deabsorption includes fixing nanozeolite molecular sieve doped with heteroatom in bodyframe structure, dipping mass spectrum stainless steel sample target plate of base material auxiliary laser deasorption in titanate coupler, placing dipped target plate in anhydrous ethyl acetate to let heteroatom nanozeolite crystal material suspension liquid be sufficiently dispersed then adding said liquid in sample-set hole of said sample target plate and baking said sample target plate.
Owner:SHANGHAI JIAO TONG UNIV
Polypeptide hormone phosphatonin
The present invention relates to a novel human protein called phosphatonin, and isolated polynucleotides encoding this protein. Also provided are vectors, host cells, antibodies, and recombinant methods for producing this human protein. The invention further relates to diagnostic and therapeutic methods useful for diagnosing and treating disorders related to this novel human protein.
Owner:UNIV COLLEGE OF LONDON +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com