Method for quantitatively analyzing tyrosine-phosphorylated proteins
A technology for quantitative analysis of tyrosine phosphorylation, applied in the field of quantitative analysis of tyrosine phosphorylated proteins, can solve the problems of inability to distinguish the occurrence of phosphorylation, and achieve the effects of saving experimental costs, convenient transformation, and clear background
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
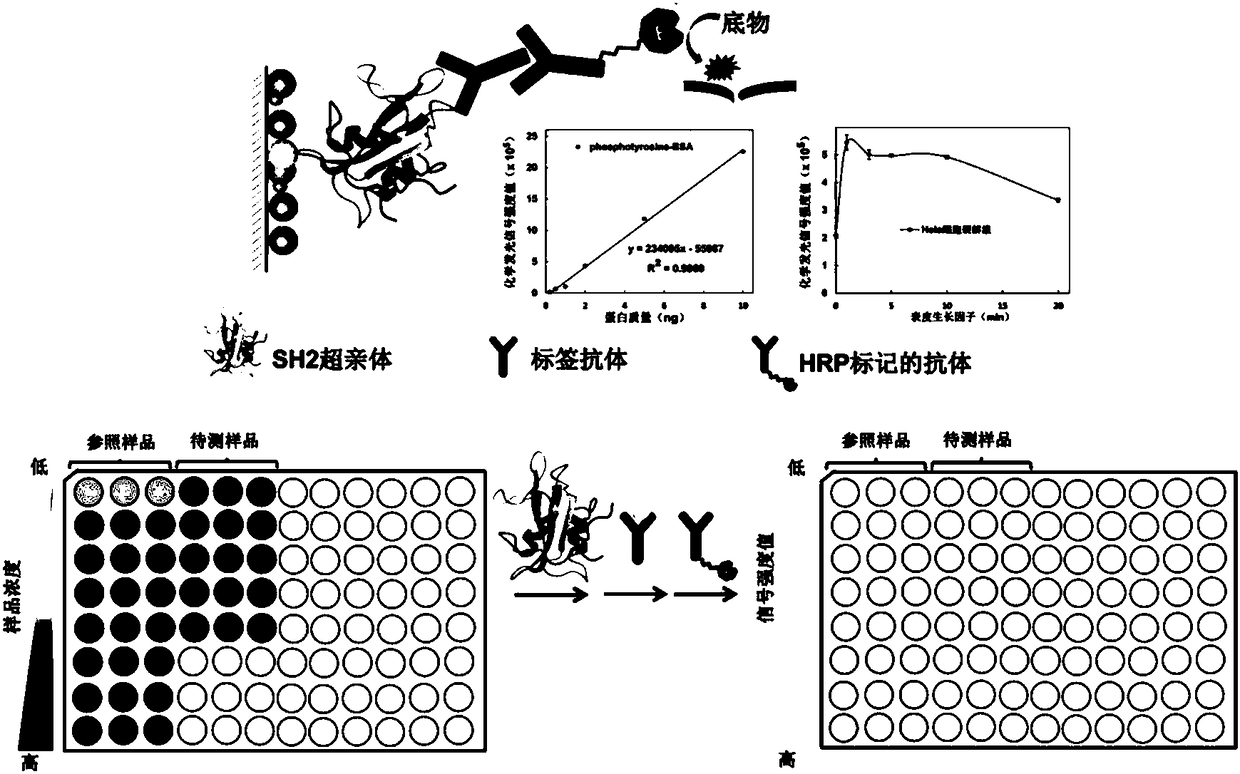
[0053] 1) Coating buffer (pH 9.6, 0.1M carbonate, freshly prepared) dilute a. tyrosine phosphorylated standard protein phosphotyrosine-BSA (SIGMA) to 0.05ng / ml, 0.25ng / ml, 0.5ng / ml ml, 1ng / ml, 2.5ng / ml, 5ng / ml, 10ng / ml, 25ng / ml, 50ng / ml, 100ng / ml, 250ng / ml, 500ng / ml, 1000ng / ml, 2500ng / ml, 5000ng / ml , b. EGF stimulated HeLa cell lysate for 1 minute to 250ng / ml, 500ng / ml, 1000ng / ml, 2500ng / ml, 5000ng / ml, c. 2000ng / ml alkaline phosphatase treatment for 0 minutes, 3 minutes, 5 minutes Minutes, 10 minutes, 20 minutes, 30 minutes, 60 minutes of HeLa cell lysate stimulated with epidermal growth factor for 20 minutes, the above samples were added to a 96-well plate at 100 μL / well, and each sample was added to three wells in parallel, at 37 degrees Celsius Incubate for 2 hours;
[0054] 2) Remove the solution in step 1), wash with TBST (20mM Tris, 150mM NaCl, pH 7.4, 0.05% (v / v) Tween-20) three times, 200μL / time, 3min / time, and finally wash in a dust-free pat dry on paper;
[0055] ...
Embodiment 2
[0069]1) Coating buffer (pH 9.6, 0.1M carbonate, freshly prepared) serially dilute tyrosine phosphorylated standard protein phosphotyrosine-BSA (SIGMA) to 0.1ng / ml, 0.25ng / ml, 0.5ng / ml , 1ng / ml, 2.5ng / ml, 5ng / ml, 10ng / ml, 25ng / ml, 50ng / ml and 50ng / ml, 100ng / ml, 250ng / ml, 500ng / ml, 1000ng / ml, 2500ng / ml, Add 5000ng / ml, 100μL / well into a 96-well plate, add each sample to three wells in parallel, and incubate at 37°C for 2 hours;
[0070] 2) Remove the solution in step 1), wash three times with TBST (20mM Tris-HCl, 150mM NaCl, pH7.4, 0.05% (v / v) Tween-20), 200μL / time, 3min / time, and finally Pat dry on a dust-free paper;
[0071] 3) Add 200 μL of blocking solution (TBST containing 5 wt.% skimmed milk powder) to the 96-well plate after being patted dry in step 2), and react at 37 degrees Celsius for 1 hour;
[0072] 4) After the reaction, wash the 96-well plate according to step 2);
[0073] 5) Add 100 μL of mouse tyrosine phosphorylated antibody P-Tyr-100 (1:1000) to the 96-well...
Embodiment 3
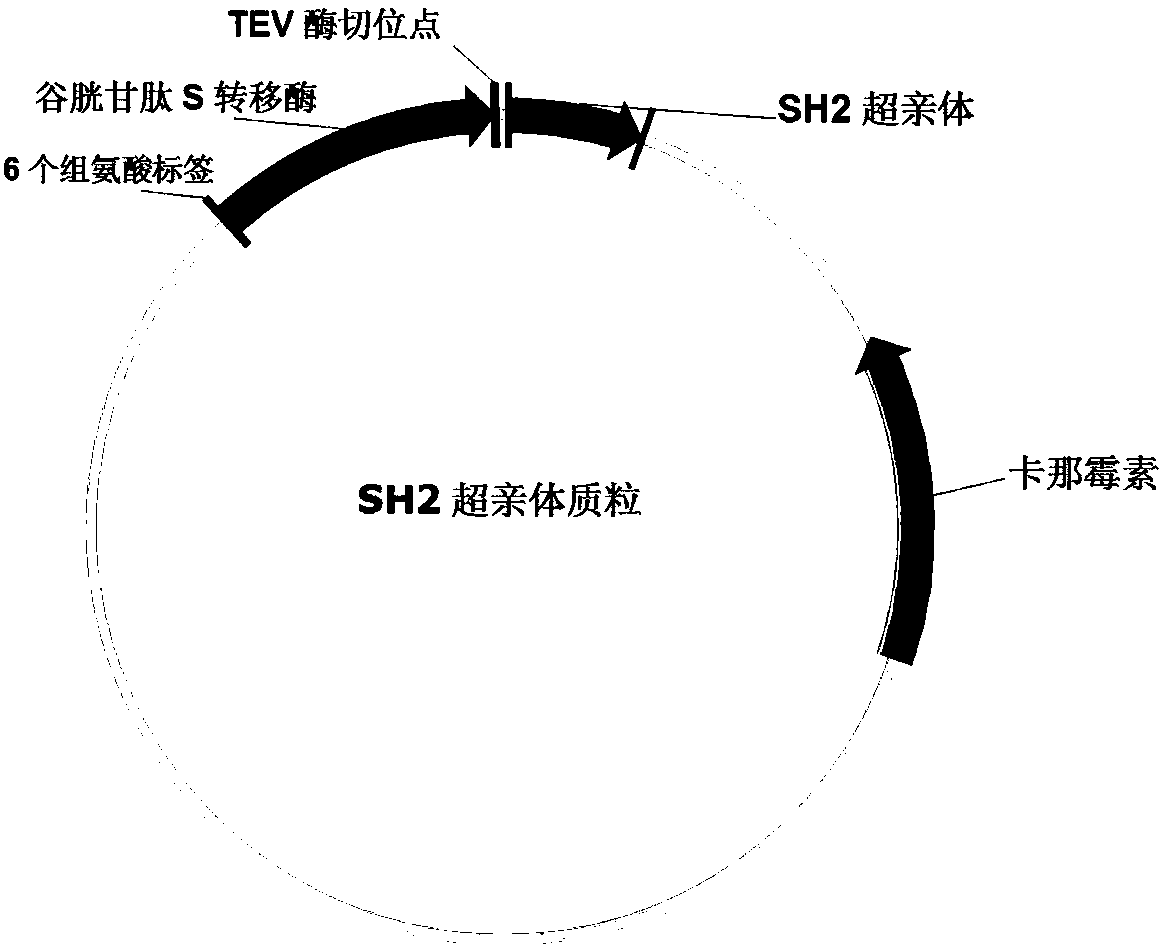
[0081] First, biotinylate the SH2 superphile, the specific operation is
[0082] 1) Take 2ml of 2mg / ml SH2 superphile and biotinylation reagent such as biotin-NHS molar ratio 1:5, shake and react in the dark, 4°C overnight; add 100μL NH 4 Cl terminated the reaction for 30min;
[0083] 2) Ultrafilter the solution after the reaction in step 2) with a 10k ultrafiltration tube, wash with PBS three times, then invert the ultrafiltration tube to collect the liquid.
[0084] 3) The concentration of the solution (SH2-biotin) after the SH2 superphile and biotin cross-linked obtained in step 3) by BCA method;
[0085] 4) The SH2-biotin solution obtained in step 3) of the HABA method measures the average number of labeled biotin per SH2;
[0086] 5) ELISA method to evaluate the best ratio;
[0087] 6) Aliquot the SH2-biotin solution obtained in step 3) and freeze at -80°C.
[0088] Secondly, use biotinylated SH2 superphile as the primary antibody to quantitatively analyze the level o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com