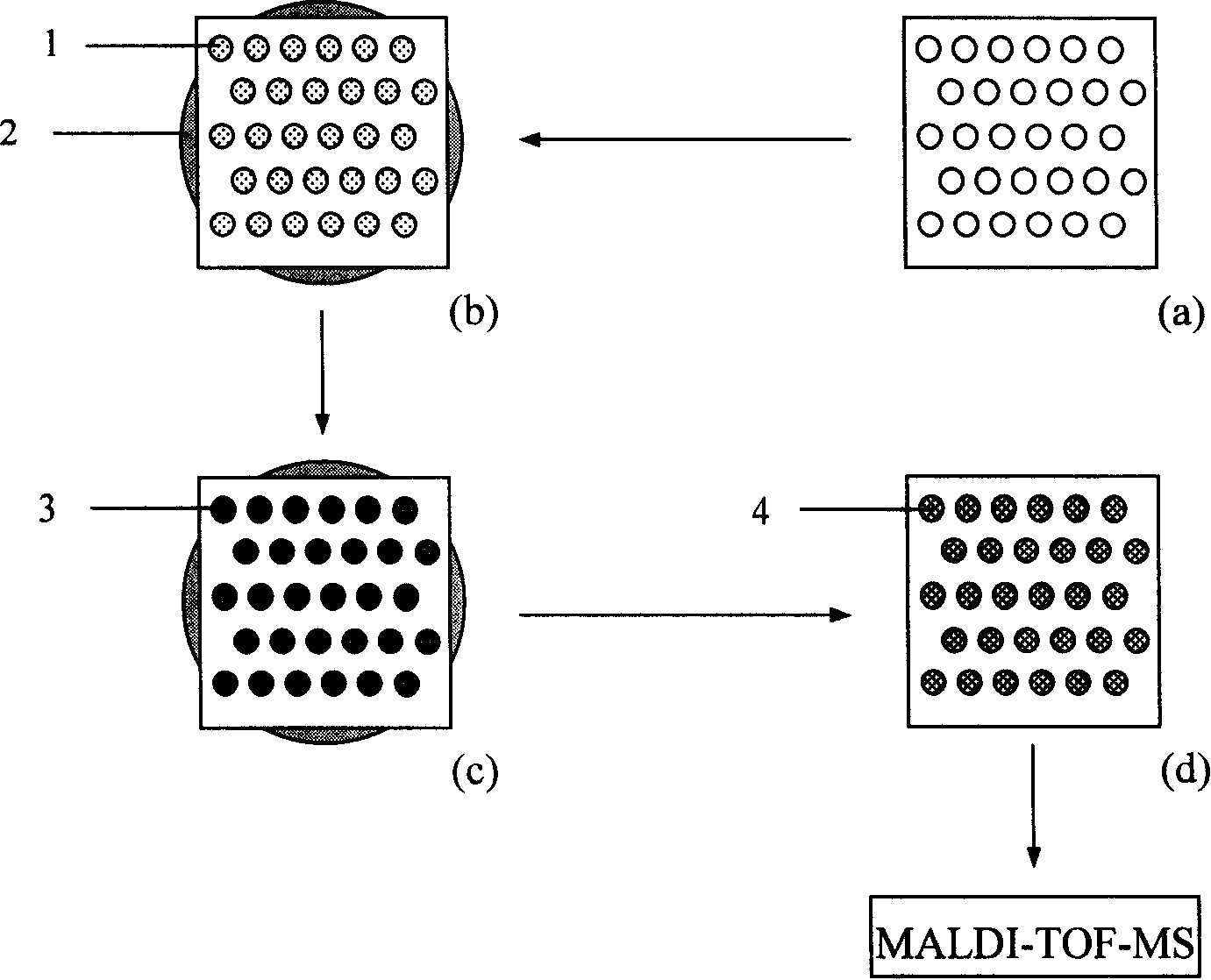
Method for fast enriching and appraising phosphopeptide on MALDI-TOF-MS sample target
A technology for enriching phosphopeptides and phosphopeptides, applied in the field of analytical chemistry, can solve the problems of large-scale proteomics, high-throughput analysis, and sample loss, and achieve the effects of convenient transformation, low cost, and loss prevention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Take a certain amount of prepared nanoparticles into a 1.5 mL centrifuge tube, add 0.15% trifluoroacetic acid solution, and sonicate for 1 minute to obtain a 0.3 mg / mL nanoparticle suspension.
Embodiment 2
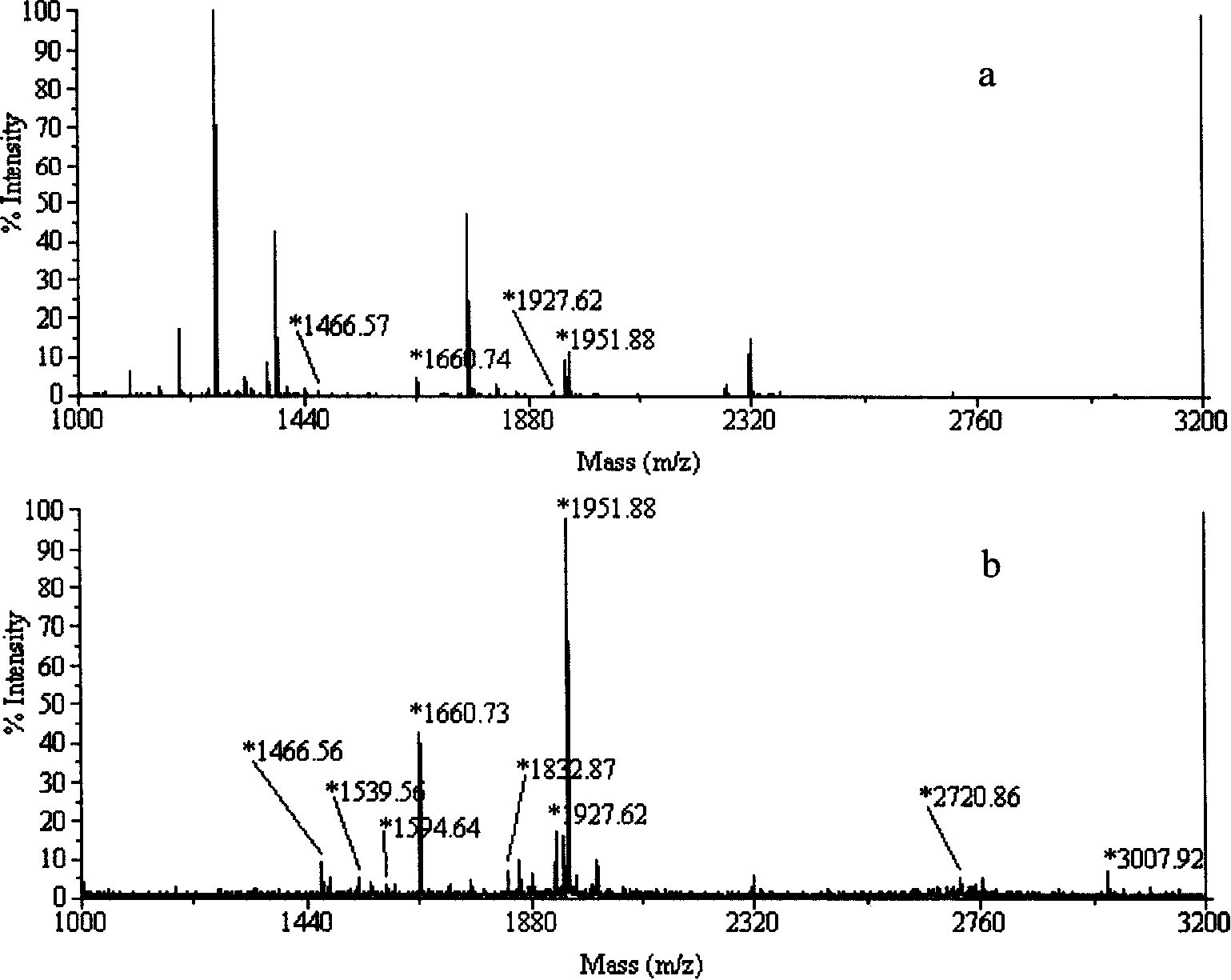
[0034]Pipette 0.8 μL (240 ng) of nano-ion suspension and distribute it on the sample hole of the stainless steel target of matrix-assisted laser desorption / ionization time-of-flight mass spectrometer (4700 proteomics Analyzer), and load 2 pmol of α-casein hydrolyzate after drying , incubate at room temperature for 10 minutes; immerse the target in a petri dish filled with 40 mL of 20% acetonitrile-0.1% trifluoroacetic acid solution, wash gently on a shaker for 15 minutes to remove non-phosphopeptides and salts, take out and rinse the target once with Millpore water , add DHB matrix (20mg / mL-DHB-%H 3 PO 4 -50% ACN) co-crystallized; MALDI-TOF-MS analysis was performed. When not enriched, there are many non-phosphopeptides, and only 3 phosphopeptides are detected, such as figure 2 a, While most of the non-phosphopeptides were removed after the enrichment of the modified MALDI target, nine phosphopeptides were detected, as figure 2 as shown in b. Detected phosphopeptides are...
Embodiment 3
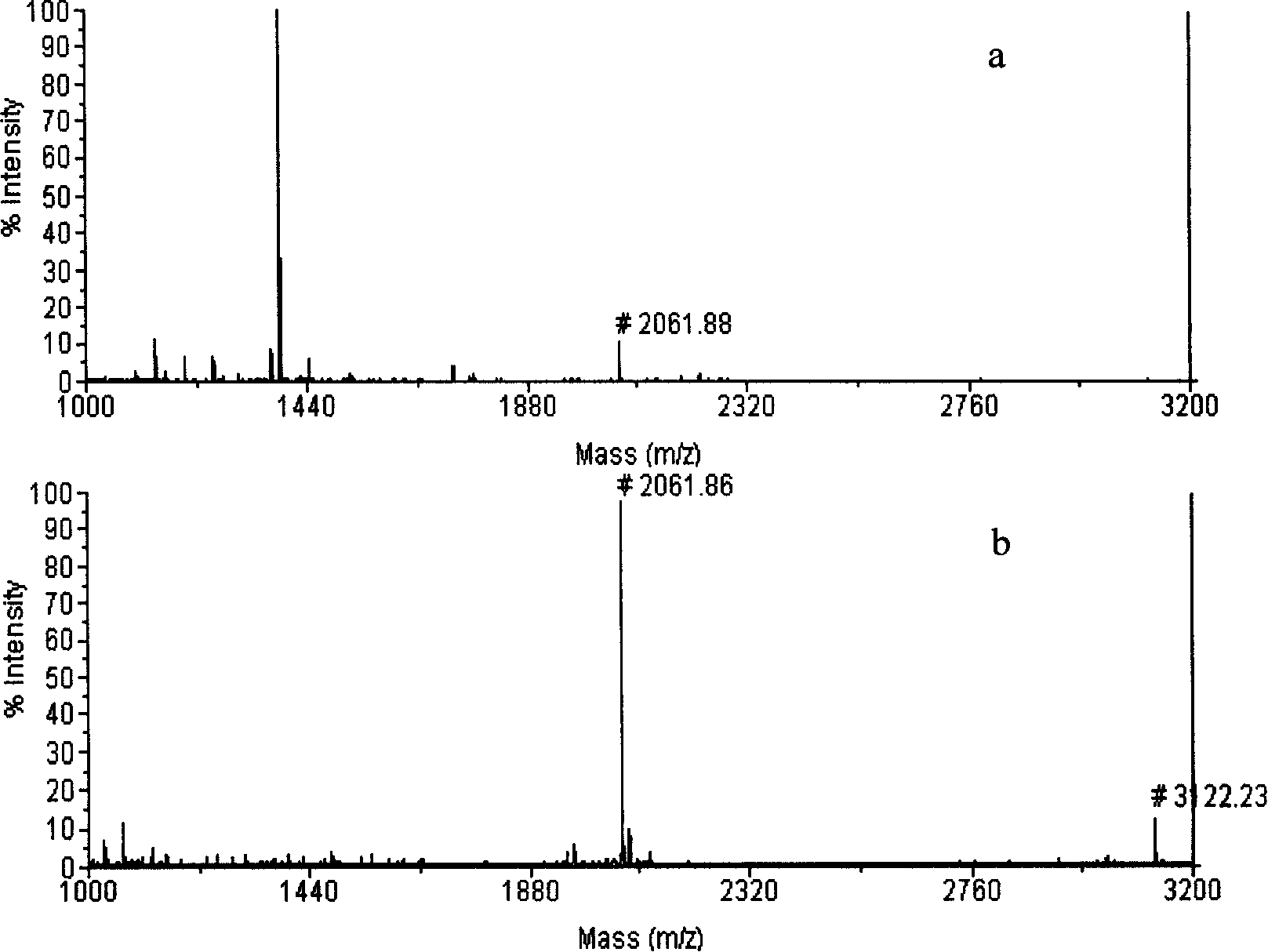
[0036] Pipette 0.8 μL of nano-ion suspension and distribute it on the sample hole of the stainless steel target of matrix-assisted laser desorption / ionization time-of-flight mass spectrometer, load 2 pmol of β-casein hydrolyzate after drying, and repeat the operation of Example 2 to enrich Collection, MALDI-TOF-MS analysis. When not enriched, only one phosphopeptide was detected, and the signal-to-noise ratio was very low. After enrichment, most of the non-phosphopeptides disappeared, and two phosphopeptides were detected, which became the two strongest peaks in the spectrum. Such as image 3 shown in a-b.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com