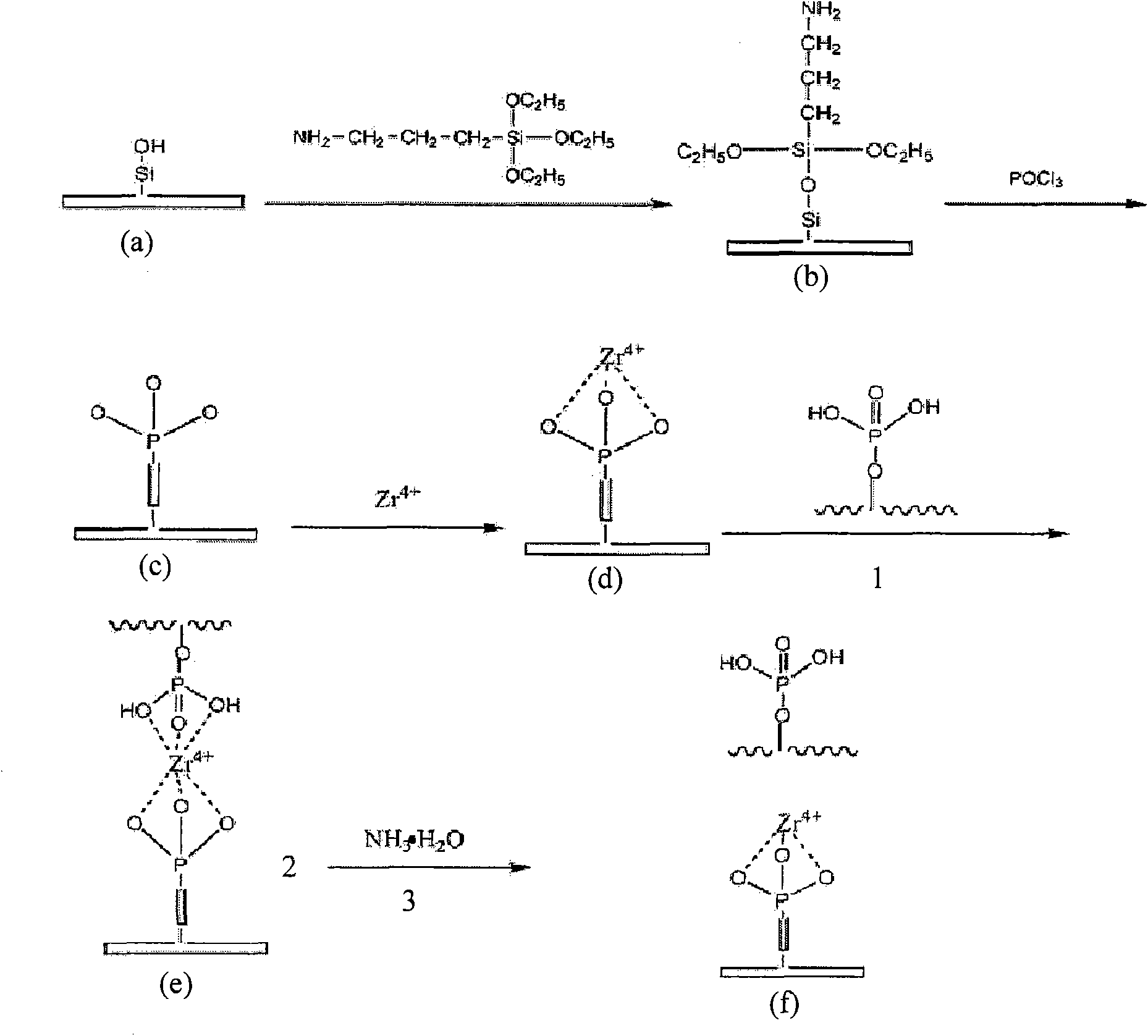
Open-tubular capillary column enriching phosphoeptide or phosphorylated protein and method
A phosphorylated protein, open-tube capillary column technology, applied in the field of analytical chemistry, can solve problems such as the inability to meet the large-scale, high-throughput analysis of proteomics, the difficulty of processing multiple samples, and the limitation of further applications, and achieve easy reuse. , Wide application range and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]Push the 0.1M HCl solution into the capillary column with a micro-sampling pump, wash it for 10 minutes at a flow rate of about 1 μL / min, then fill the capillary with HCl solution, seal the end with a rubber stopper, and let it stand at room temperature for 30 minutes. Push out the HCl solution, rinse with water until the pH of the effluent is about 7, inject 0.1M NaOH solution into the capillary, rinse at a flow rate of about 1 μL / min for 10 minutes, then fill the capillary with NaOH solution, seal the end with a rubber stopper, and let it stand at room temperature 2h. Push out the NaOH solution and rinse with water until the pH of the effluent is about 7.
[0030] Push 10% 3-aminopropyltriethoxysilane (APES) methanol solution (v / v) into the capillary, seal both ends of the capillary, and immerse the capillary in a 70° C. water bath for 8 h. Then push out the APES solution, rinse the capillary with 20 μL of methanol solution, and dry it with nitrogen gas.
[0031] Qui...
Embodiment 2
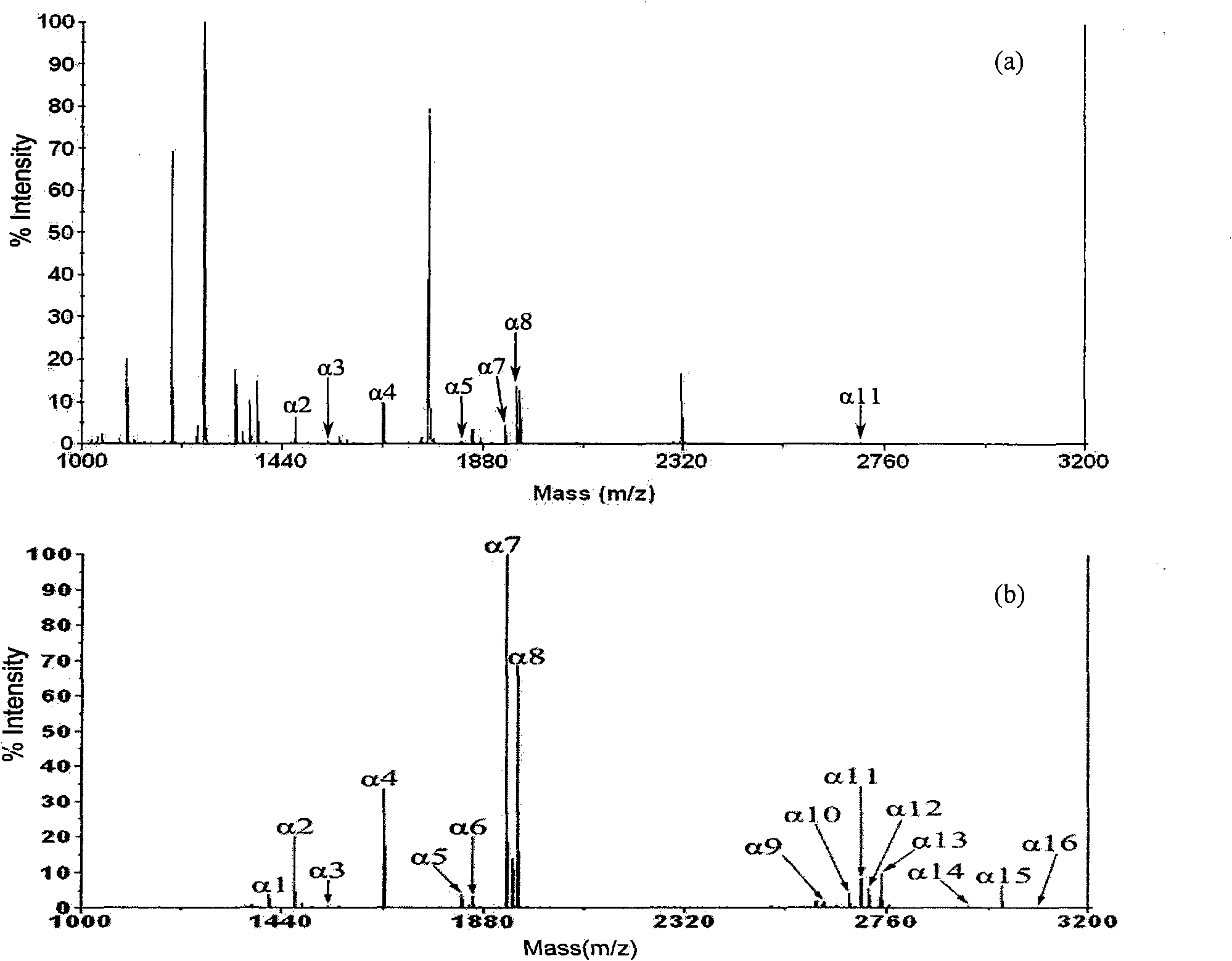
[0034] After diluting the digestion solution of the standard phosphorylated protein α-casein with 50% ACN, 0.1% TFA solution to 2 pmol / μL, use a micro-sampling pump to push into the zirconium phosphate group-modified opening at a flow rate of 0.2 μL / min. A total of 20 μL was injected into a capillary column (inner diameter 50 μm, length 150 cm). Wash the inner wall of the capillary column successively with 50 μL of 50% ACN, 0.1TFA% solution and 50 μL of pure water at a flow rate of 1.0 μL / min to remove impurities such as non-phosphopeptides and salts adhering to the inner wall. Inject 10 μL of 0.1M ammonia solution into the capillary column at a flow rate of 0.2 μL / min to elute the specifically adsorbed phosphopeptides, spot the eluted solution on the MALDI-TOF target, and after natural air drying, spot the DHB solution (50% ACN , 0.1%H 3 PO 4 ), followed by mass spectrometry analysis. When not enriched, many non-phosphopeptides appeared, and only 7 phosphopeptides were det...
Embodiment 3
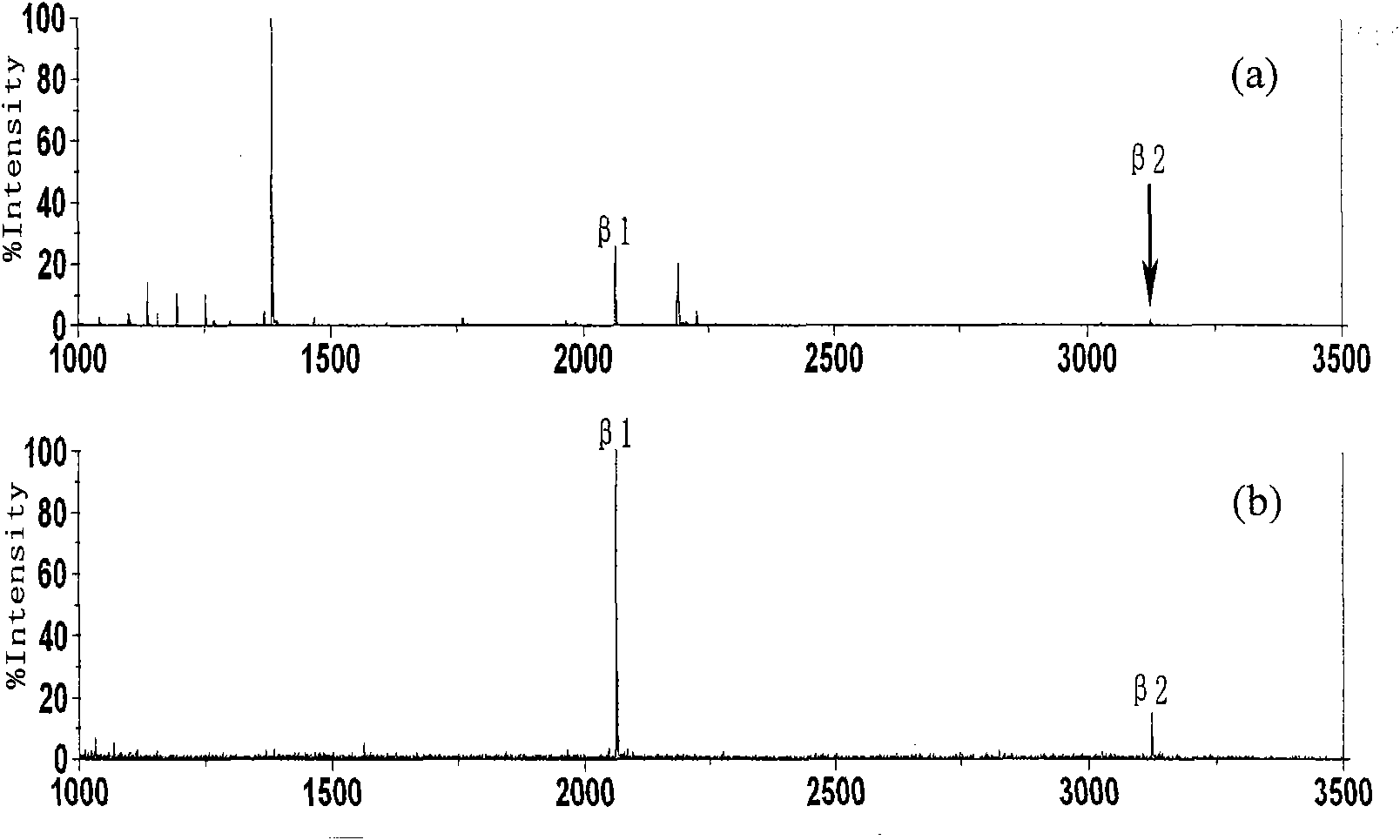
[0036] The zirconium phosphate group-modified open-tube capillary column was used to load the β-casein hydrolyzate, and the operation in Example 2 was repeated for enrichment and MALDI-TOF-MS analysis. When not enriched, the signal-to-noise ratio is very low, but after enrichment, most of the non-phosphopeptides disappear, and two phosphopeptides are detected, which become the two strongest peaks in the spectrum, such as image 3 shown in a-b.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com