Method for specifically separating and enriching phosphorylated peptide and glycosylated peptide
A technology for separation, enrichment, and phosphorylation of peptides, which is applied to peptide preparation methods, chemical instruments and methods, and peptides. The effect of large specific surface area, good enrichment capacity, good magnetic response and hydrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
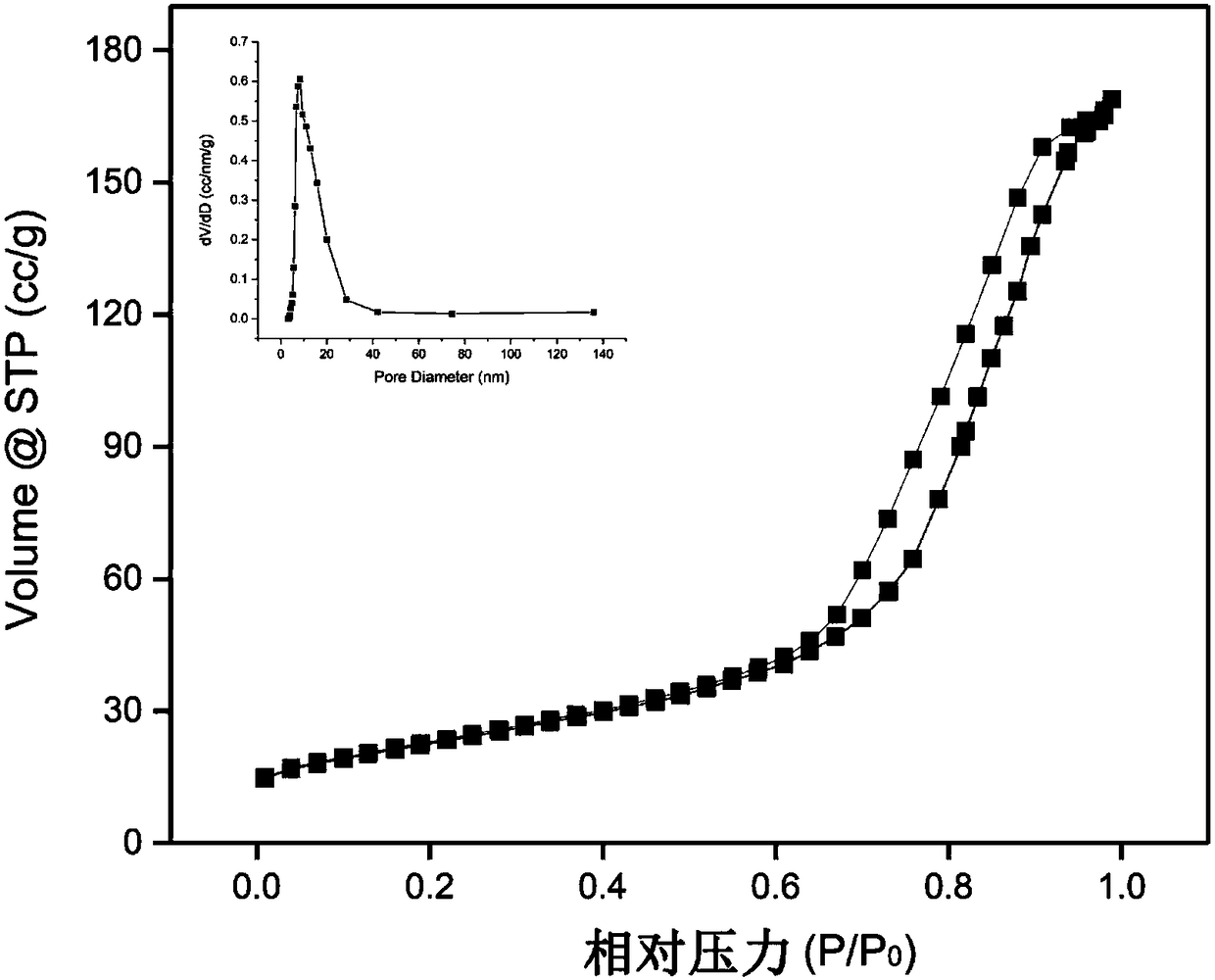
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of hydrophilic magnetic mesoporous titanium dioxide material
[0032] (1) Add 1.35 g FeCl 3 ·6H 2 After O is magnetically stirred in 75 mL of ethylene glycol until the solution is clear, add 3.6 g of NaAc, then transfer to a hydrothermal reactor after fully stirring and sonicating, heating at 200°C for 12 hours, and deionizing after the reactor is cooled Wash the product with water and ethanol three times, and dry it in vacuum at 50℃;
[0033] (2) Disperse 50 mg of the product obtained in (1) ultrasonically in 400 ml ethanol containing 4 ml ammonia, add 2.5 ml n-butyl titanate dropwise, and react the resulting mixed solution at 30 ℃ for 24 h. Wash thoroughly with deionized water and absolute ethanol;
[0034] (3) Transfer the product obtained in (2) to a mixed solution containing 60 ml of ethanol and 30 ml of deionized water after ultrasonic dispersion, then transfer it to a hydrothermal reactor and heat it at 160°C for 12 h;
[0035] (4) The product obtai...
Embodiment 2
[0040] Example 2: The hydrophilic magnetic mesoporous titanium dioxide material obtained in Example 1 was used as a solid-phase adsorbent for the separation and enrichment of glycopeptides in glycoprotein HRP enzymatic hydrolysis products
[0041] (1) Sample preparation: 1 mg HRP in 50 mM NH 4 HCO 3 Enzymatic hydrolysis in the solution at 37°C for 16 h.
[0042] (2) 150 μg of hydrophilic magnetic mesoporous titanium dioxide material was dispersed in 100 μL of loading buffer containing 100 fmol / μL of the HRP digestion product of step (1), and incubated at 37°C for 20 min. Rinse the sample three times with 200 μL of loading buffer. Use 8 μL ACN / H 2 O / TFA (50 / 49 / 1, v / v / v) was eluted for 30 min.
[0043] The loading buffer is a buffer solution containing 85% by volume of acetonitrile and 5% by volume of trifluoroacetic acid.
[0044] (3) Mass spectrometry analysis: Take 1 μL of the eluent in step (2) and spot the target, dry it naturally, and perform mass spectrometry analysis. The mass ...
Embodiment 3
[0046] Example 3: The hydrophilic magnetic mesoporous titanium dioxide material obtained in Example 1 is used as a solid-phase adsorbent for the separation and enrichment of phosphorylated peptides in the hydrolyzed product of phosphorylated protein β-casein
[0047] (1) Sample preparation: 1 mg β-casein in 50 mM NH 4 HCO 3 Enzymatic hydrolysis in the solution at 37°C for 16 h.
[0048] (2) 150 μg of hydrophilic magnetic mesoporous titanium dioxide material was dispersed in 100 μL of loading buffer containing 100 fmol / μL of the β-casein enzymatic hydrolysis product of step (1), and incubated at 37°C for 20 min. Rinse the sample three times with 200 μL of loading buffer. Elute with 8 μL 0.4 M ammonia water for 30 min.
[0049] (3) The loading buffer is a buffer solution containing 95% by volume of acetonitrile and 0.1% by volume of trifluoroacetic acid.
[0050] (4) Mass spectrometry analysis: Take 1 μL of the eluent in step (2) and spot the target, dry it naturally and perform mass s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com