Fine purification method of polyvalent anti-snake venom freeze-dry blood serum
An anti-snake venom and freeze-drying technology, which is applied in the direction of antibodies, skin diseases, and drug combinations, can solve the problems of low purity and low antibody titer, and achieve the effects of low foreign protein, good drug safety, and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
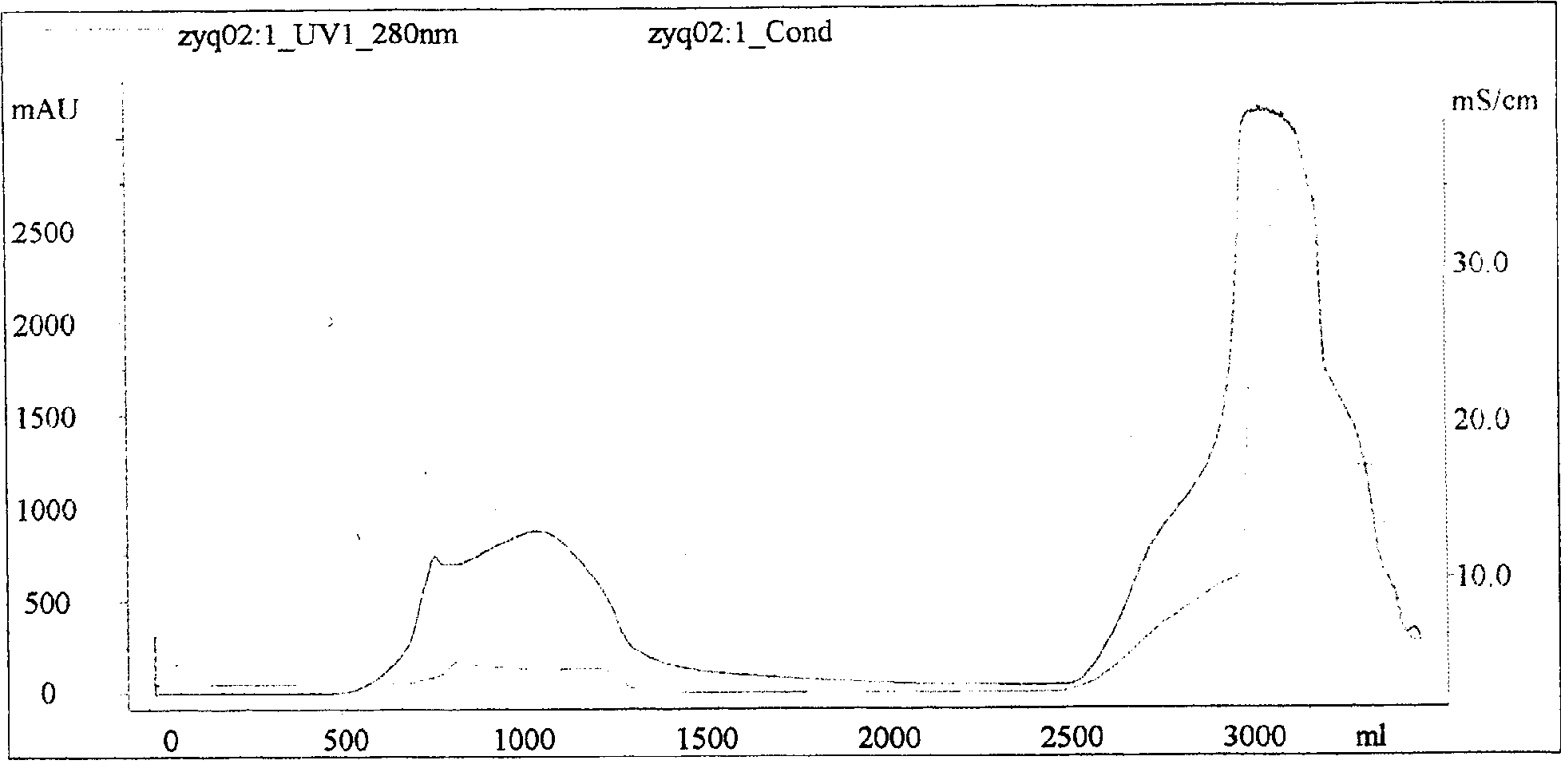
[0020] (1) Preparation of antiserum of the present invention
[0021] 1. Preparation of antigen
[0022] The venoms of five-step snake, pit viper, iron head, bamboo leaf green, viper, coral snake, golden krait, cobra, and king cobra were obtained by live snake bite and extrusion method respectively. The fresh venom is immediately vacuum-dried with phosphorus pentoxide or anhydrous calcium chloride, and multiple batches of dried snake venom are mixed according to snake species, and stored in brown bottles for frozen storage.
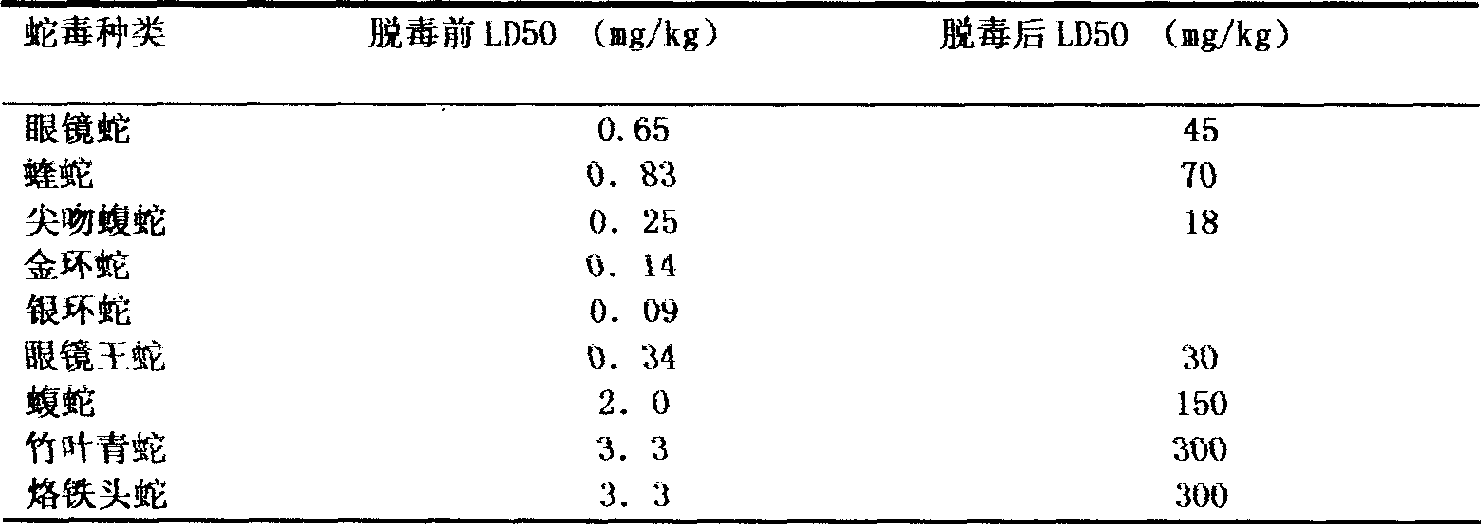
[0023] Determination of toxicity: The Blliss simplified probability unit method is used to determine the toxicity of snake venom, and it is expressed as the half-dead dose LD50. Randomly select 18-20 grams of mice to form several groups, with more than 6 mice in each group, and adjust the dose after pre-testing. The mortality rate of the highest dose group is close to 100%, the mortality rate of the lowest dose group is 0%, the ratio between doses is con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com