Recombinant FAP binding proteins and uses thereof
A technology for binding proteins and ankyrins, which can be applied in drug combinations, immunoglobulins, peptide/protein components, etc., and can solve problems such as incomplete understanding of potential effects, complex effects, and incomplete understanding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0236] Example 1: Selection of binding proteins comprising an ankyrin repeat domain with binding specificity for FAP
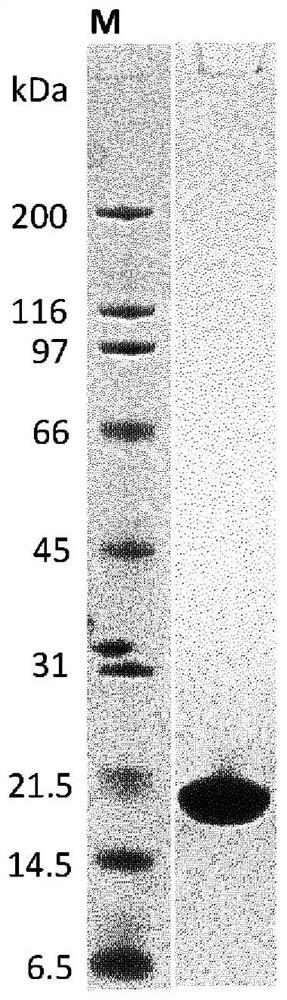
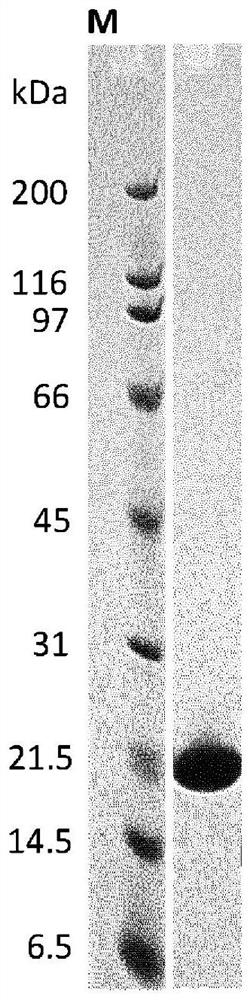
[0237] Using ribosome display (Hanes, J. and Plückthun, A., PNAS 94, 4937-42, 1997), similarly from A number of ankyrin repeat proteins with binding specificity for human FAP (hFAP) were selected from the library. Binding of selected clones to recombinant human FAP targets was assessed by homogeneous time-resolved fluorescence (HTRF) of crude extracts, demonstrating the successful selection of hundreds of hFAP-specific binding proteins. For example, the ankyrin repeat domain of SEQ ID NO: 1 to 33 constitutes the amino acid sequence of a selected binding protein comprising an ankyrin repeat domain with binding specificity for hFAP. Individual ankyrin repeat modules from such ankyrin repeat domains with binding specificity for hFAP are provided in SEQ ID NOs: 48 to 134.
[0238] Selection of FAP-specific ankyrin repeat proteins by ribosome display
[023...
Embodiment 2
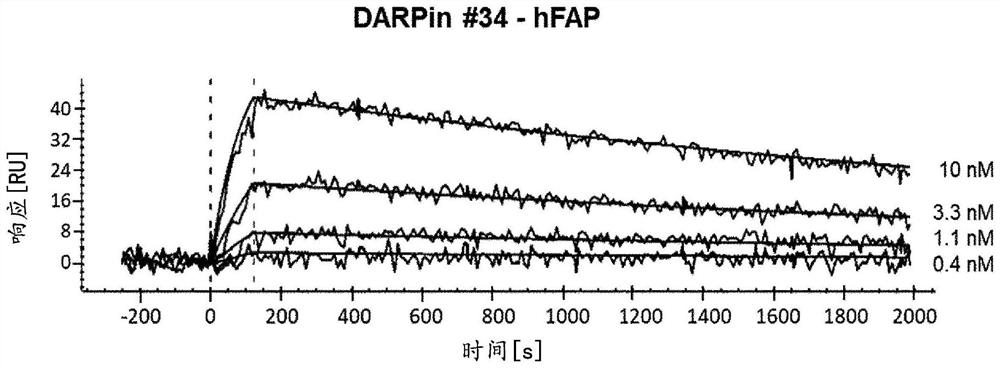
[0293] Example 2: Determination of Ankyrins with Binding Specificity for FAP by Surface Plasmon Resonance (SPR) Analysis The dissociation constant (K D )
[0294] The binding affinity of the purified ankyrin repeat protein to the human FAP target was analyzed using the ProteOn instrument (BioRad) and measured according to standard procedures known to those skilled in the art.
[0295] Briefly, human FAP was diluted in 10 mM sodium acetate pH 5.3 buffer and covalently immobilized on a GLC chip (BioRad) to a level of approximately 2000 resonance units (RU). Then by injecting 200 μl of running buffer (PBS, pH 7.4, containing 0.005% Tween ) (on-rate measurement) followed by injection of running buffer flow at a constant flow rate of 100 μl / min for at least 25 minutes (off-rate measurement) to measure the interaction of ankyrin repeat protein and hFAP. Use 15 μl of 10 mM glycine (pH 2), followed by 15 μl of 124 mM H 3 P0 4 to regenerate. The signal (i.e., RU value) betwee...
Embodiment 3
[0300] Example 3: Determination of FAP-specific ankyrin repeat protein binding on FAP+ cells by FACS
[0301] Binding of purified FAP-specific ankyrin repeat protein to FAP-expressing cells was analyzed by FACS.
[0302] Using a multi-well plate, add 20,000 WI38 cells per well in 50 μl PBS. 50 μl of appropriate dilutions of ankyrin repeat protein (2x dilution steps) were then added to the cells and incubated on ice for 30 minutes. After the ankyrin repeat protein binding reaction, the cells were washed twice with PBS. Direct immunofluorescence detection of His-tagged ankyrin repeat protein using Penta-His AF647 conjugate (QIAgen) and direct immunofluorescence detection using LIVE / DEAD were then performed together in one step. TM Live cell assay with Fixable Green Dead Cell Stain Kit (ThermoFisher). Combine Penta-His AF647 conjugate with LIVE / DEAD TM Fixable green dye was co-applied to cells at 1 / 200 (Penta-His) and 1 / 1000 (L / D Fixable) final dilutions and incubated on ic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com