Device and method for integrated diagnostics with multiple independent flow paths
a technology of integrated diagnostics and flow paths, applied in specific use bioreactors/fermenters, instruments, biomass after-treatment, etc., can solve the problems of unidirectional flow, different wash and substrate reagents, and inability to use enzyme amplified assays, etc., to achieve the control of the delivery of clean wash solution, reduce non-specific binding, and increase assay performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
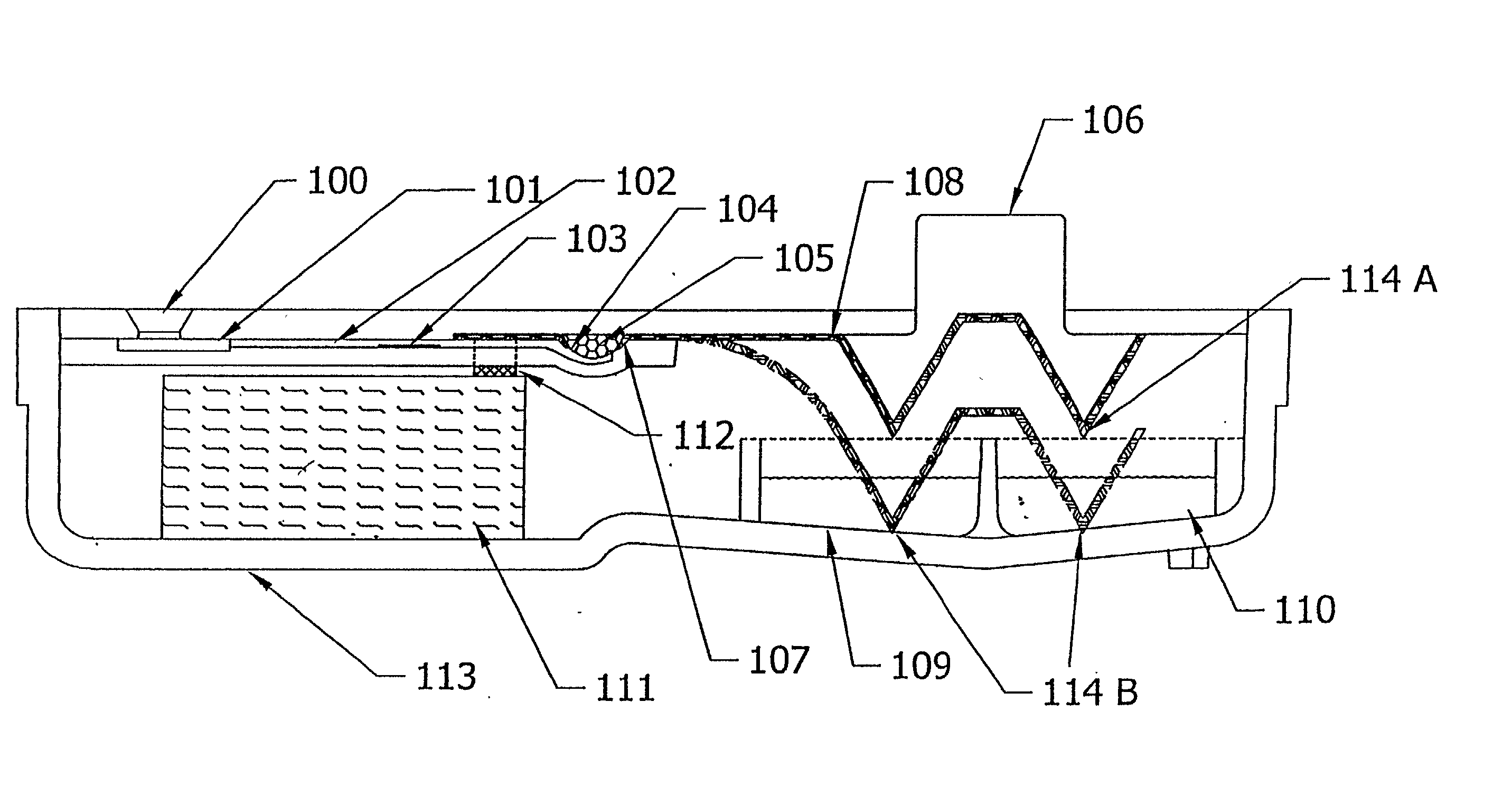
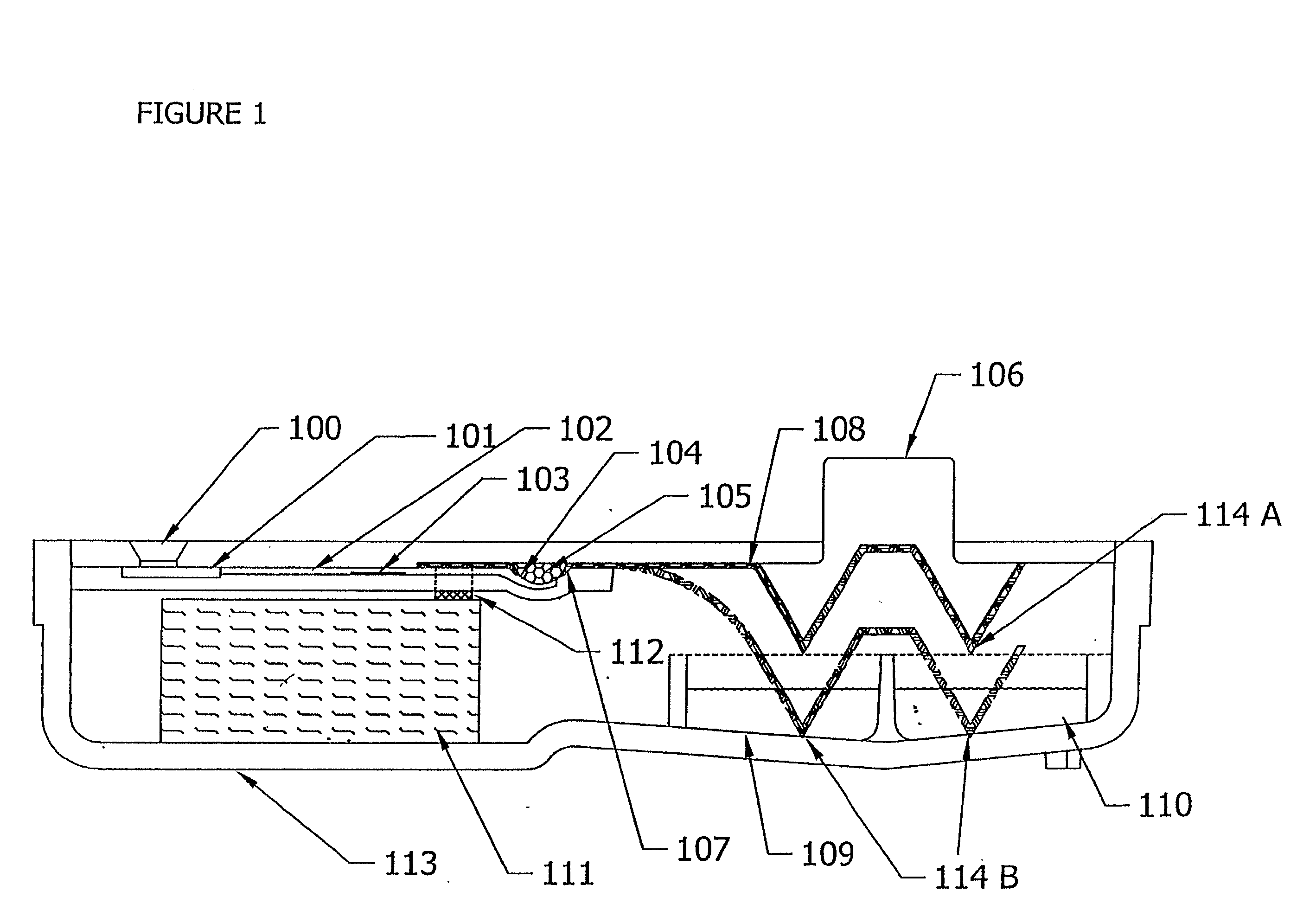
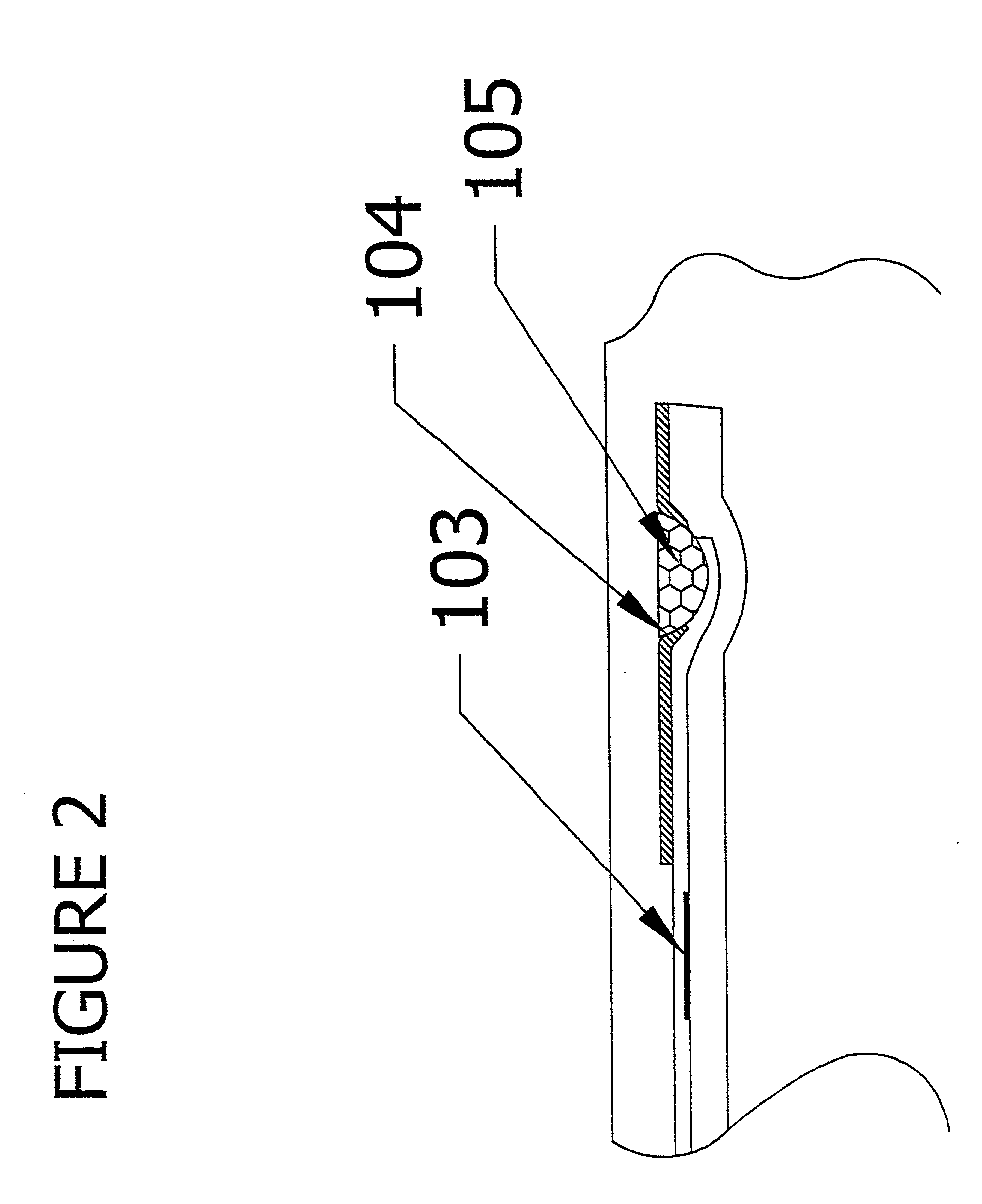
[0159] The device of example 1 can be simplified by use of a fluorescent label or colored particle e.g. colloidal gold, attached to the chicken anti-heartworm antibody instead of using HRP as the conjugate. In this case, the substrate reagent can be omitted, and the signal is viewed after the unbound reagent is washed away.
example 3
[0160] To detect multiple analytes in a single sample, a multi-channel device is constructed from the device in example 1 by feeding multiple sample delivery channels from a single sample entry port (FIG. 7). Each sample delivery channel will contain different conjugate binding reagents to detect the different analytes. Thus, four solid-phase containers are constructed to be adjacent to each other. The assembly is attached to a plastic part with four sample delivery channels.
PUM
| Property | Measurement | Unit |
|---|---|---|
| widths | aaaaa | aaaaa |
| widths | aaaaa | aaaaa |
| lengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com