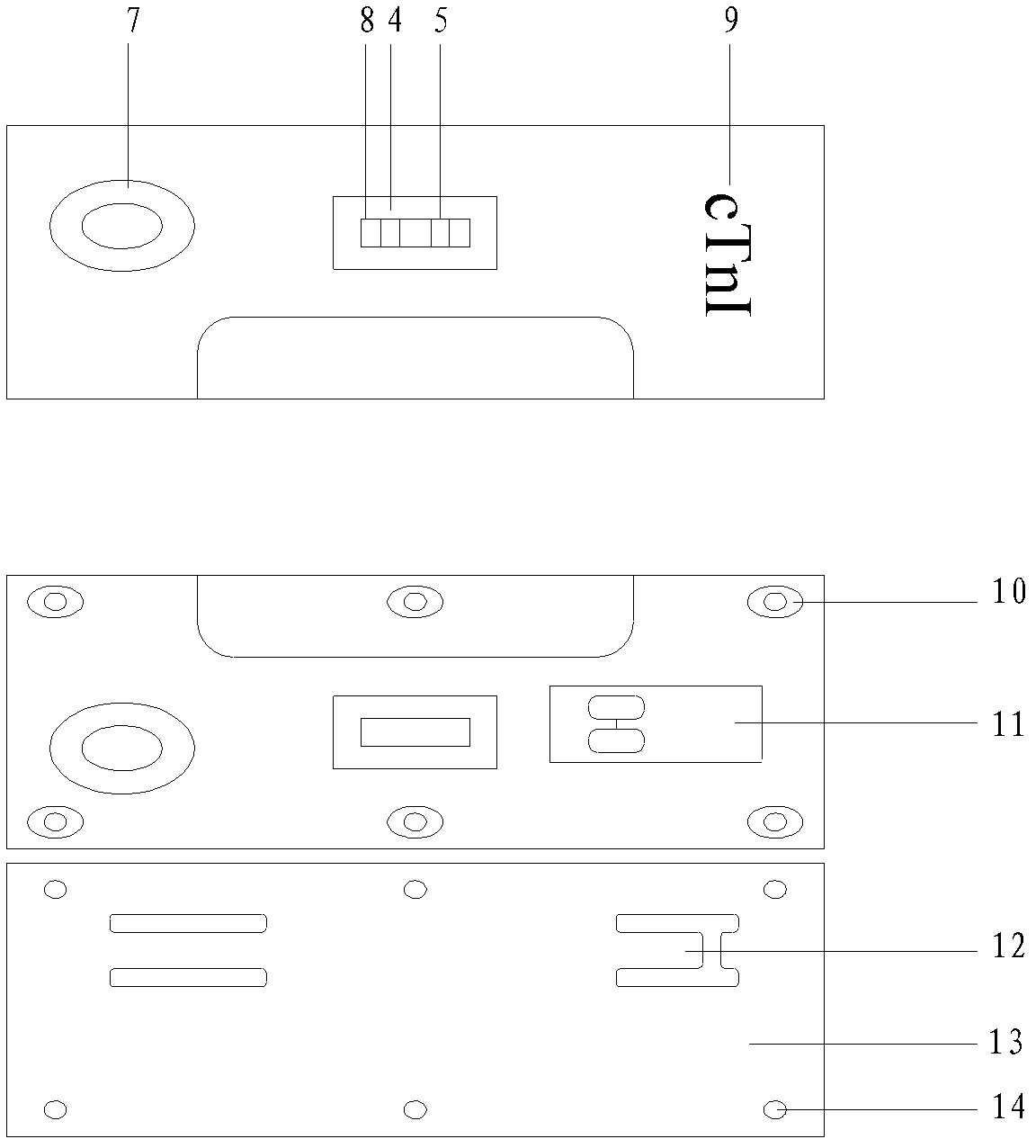Immune fluorescent test strip component for quickly quantitatively detecting troponin I, detection card component comprising immune fluorescent test strip component and preparation methods for immune fluorescent test strip component and detection card component
A technique for quantitative detection of troponin, which is applied to the immunofluorescence test strip assembly for rapid quantitative detection of troponin I and its preparation, the detection card assembly supported by the immunofluorescence test strip assembly and the field of preparation thereof, which can solve the problem of Strong applicability, unable to meet the problems of short detection time, etc., to achieve the effect of removing matrix effect, improving reaction sensitivity and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
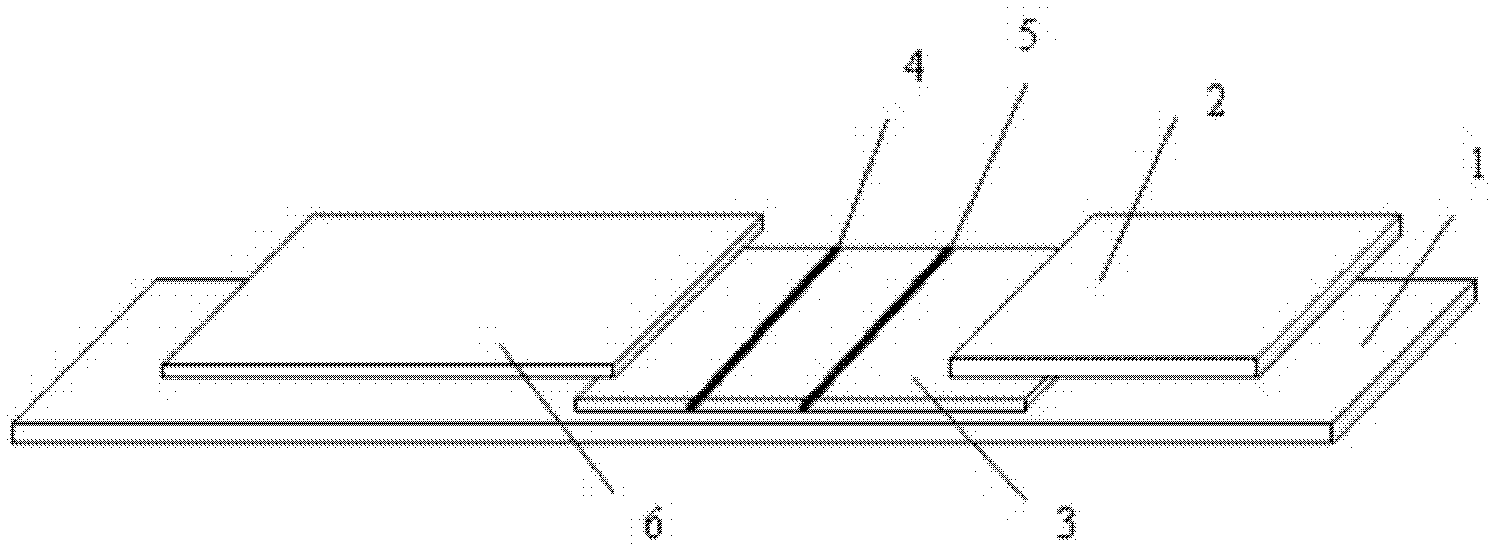
[0055] as attached figure 1 As shown, an immunofluorescence test strip assembly for rapid quantitative detection of troponin I includes a test strip and a platinum porphyrin-labeled specific antibody that is used in conjunction with the test strip and is independently packaged; wherein the test strip is made of a substrate 1 and the sequentially bonded water-absorbent pad 2, coated analysis membrane 3, and sample pad 6 arranged on the substrate 1; the coated analysis membrane 3 is provided with a detection line 4 and a quality control line 5; the detection line 4. The specific antibody coated is anti-troponin I monoclonal antibody, and the quality control line 5 is coated with specific antibody as anti-rabbit IgG antibody;
[0056] Wherein, the surface of the bottom liner 1 is coated with glue or double-sided tape, which is used to fix the water-absorbing pad 2, the coated analysis film 3 and the sample pad 6, specifically, the coated analysis film 3 is adhered to the bottom ...
Embodiment 2
[0097] The preparation method of the present embodiment is basically the same as that of the first embodiment, the difference is that:
[0098] In step 2, the method for preparing the coating buffer of detection line 4: use 50 mM Tris buffer with pH value of 7.6, which contains 0.8% methanol, 0.6% bovine serum albumin and 1 mg / ml 19C7 antibody.
[0099] The preparation method of the quality control line coating buffer: use 50mM PB buffer solution with a pH value of 7.6, and the PB buffer solution contains 0.7% methanol, 0.5% bovine serum albumin and 0.5mg / ml rabbit IgG antibody.
[0100] Adjust the BIO-DOT film spraying machine so that the film liquid volume is 20ul / 40cm, and put the test strip after spraying the NC film 3 into a vacuum drying oven at 25°C-37°C for drying for 1.5 hours.
Embodiment 3
[0102] The preparation method of the present embodiment is basically the same as that of Example 1, the difference is:
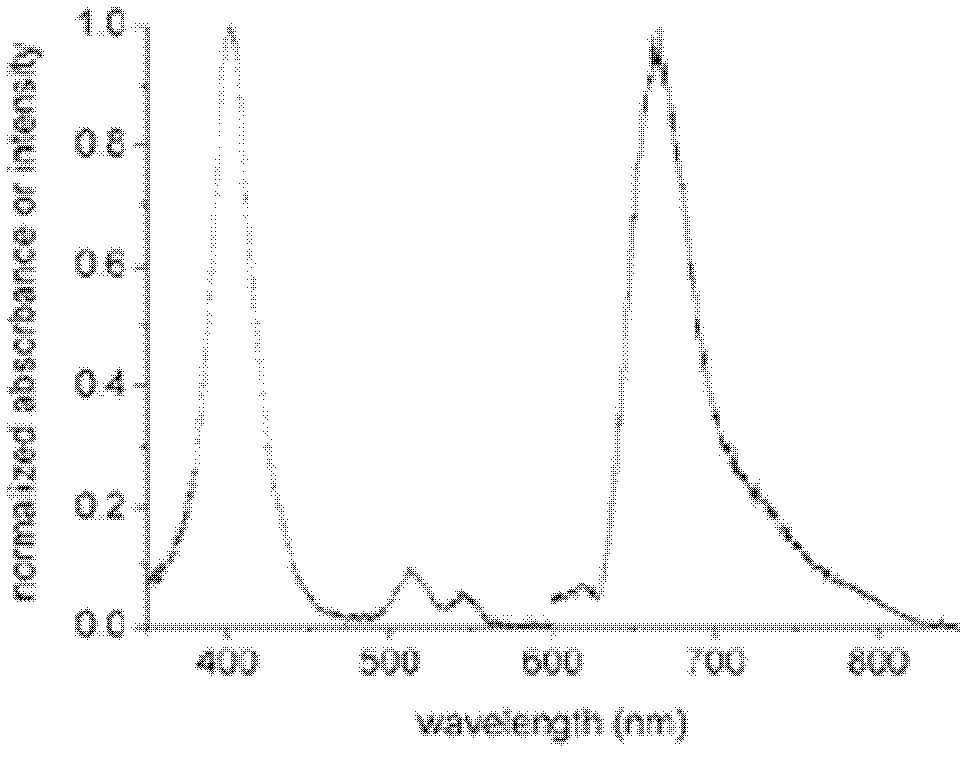
[0103] In step 3, use 0.1M sodium bicarbonate solution to dilute the concentration of anti-troponin I monoclonal antibody and anti-rabbit IgG antibody to 1mg / ml respectively, take 5ml antibody solution respectively, add 40mg fluorescein platinum porphyrin solution respectively and stir well.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com