Fluorescent immune chromatographic test strip for quantitively detecting troponin I and preparation method thereof
A fluorescent immunochromatography, troponin technology, applied in biological testing, measuring devices, analytical materials, etc., can solve problems such as expensive equipment, unsuitable for single-person and small batch detection, and inability to achieve accurate quantification. Simple operation, shorten the detection time, and improve the effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
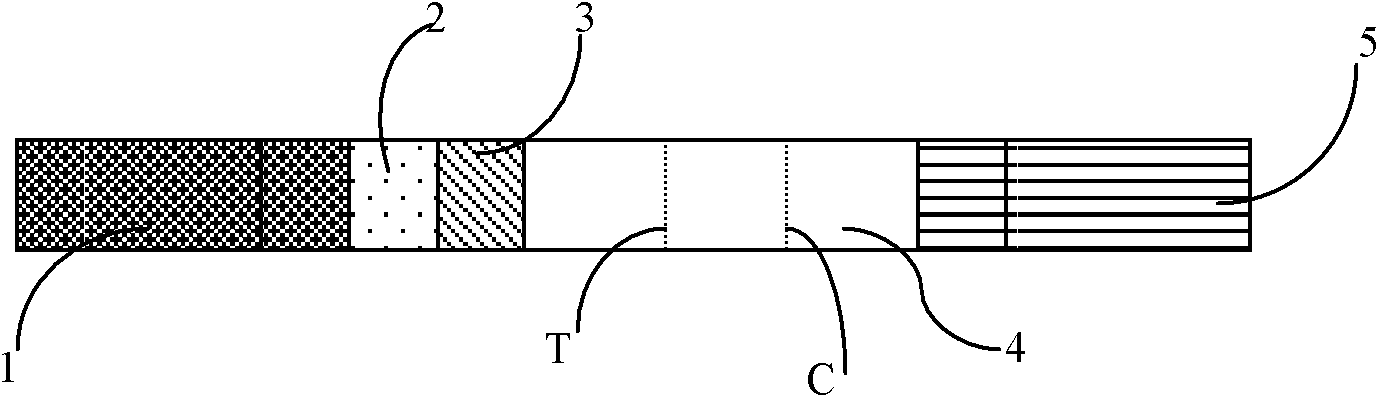
[0033] The preparation of the fluorescent immunochromatography test strip of embodiment 1 troponin I (referring to Fig. 1)
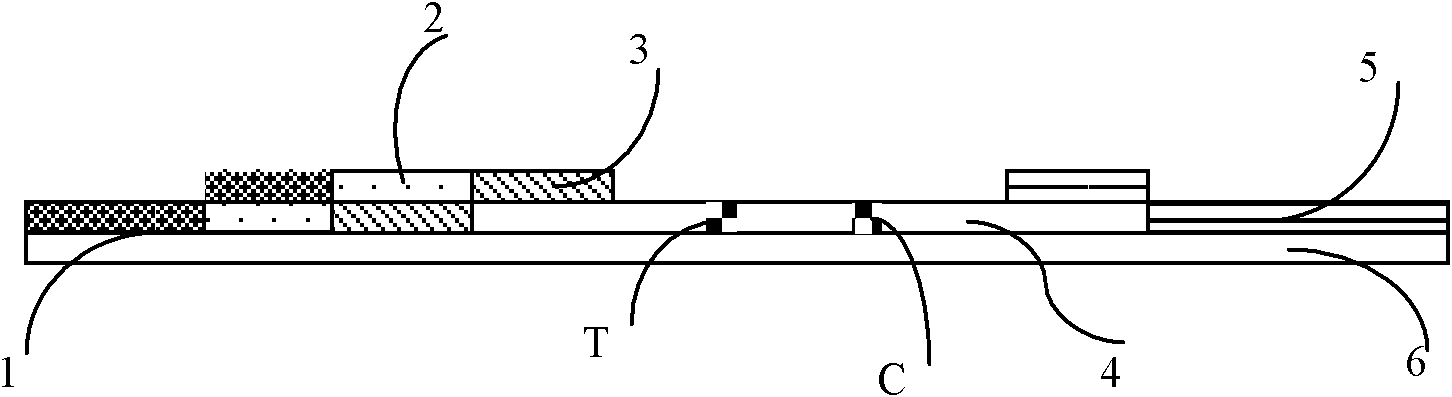
[0034]Referring to accompanying drawing 1, the fluorescent immunochromatography test strip of troponin I of the present embodiment comprises the nitrocellulose membrane 4 that is coated with quality control band (C) and detection band (T) on base plate 6, base plate, High-strength absorbent paper covering one side of the nitrocellulose membrane, 5 monoclonal antibody pads of fluorescently labeled troponin I covering the other side of the nitrocellulose membrane, 2 monoclonal antibody pads of biotin-labeled troponin I Clone antibody pad 3 and sample pad 1;
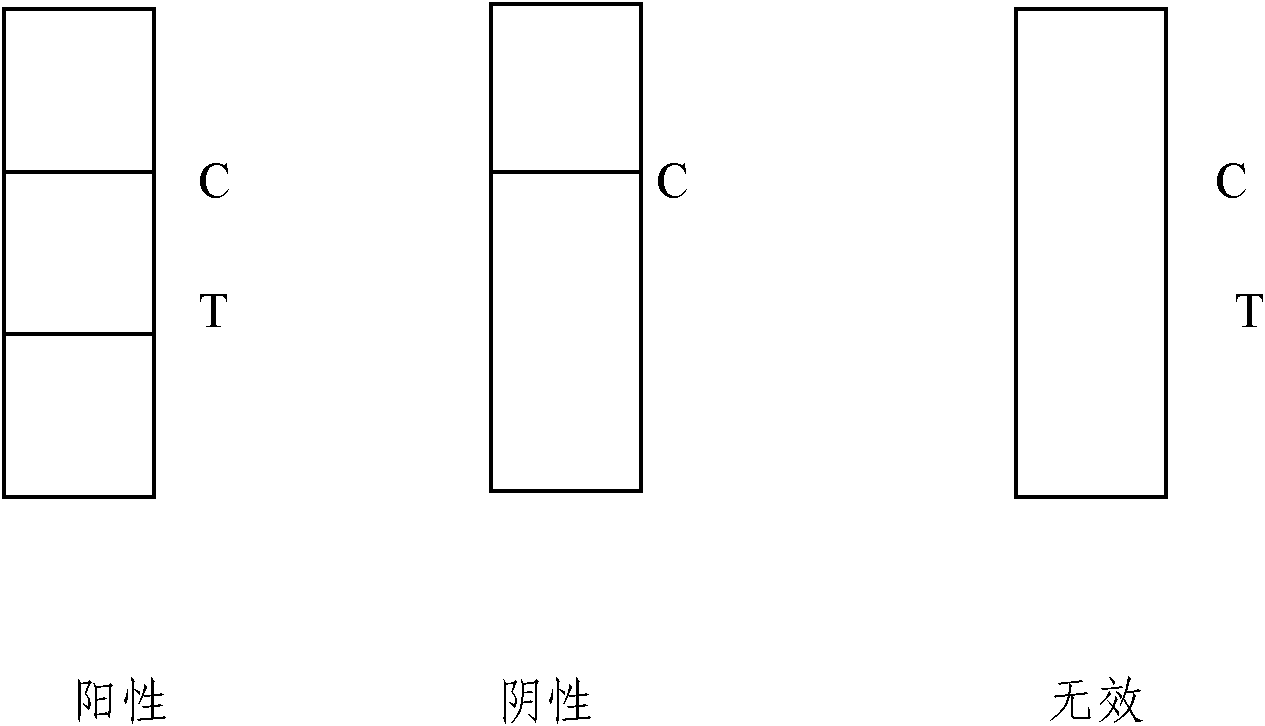
[0035] The detection zone T is coated with streptavidin; the quality control zone is coated with rabbit anti-mouse IgG antibody.
[0036] The test strip preparation method of the present embodiment comprises the following steps:
[0037] A. Antibody preparation: select commercial troponin I monoclon...
Embodiment 2
[0048] Except for the preparation step of the fluorescent microsphere mat labeled with troponin I monoclonal antibody: fluorescent microspheres with a diameter of 210 nm were selected, and other steps were the same as in Example 1.
Embodiment 3
[0050] Except that in the preparation step of the fluorescent microsphere pad labeled troponin I monoclonal antibody, the amount of 4 μl / cm was sprayed on the fluorescent microsphere pad, other steps were the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com