Procalcitonin detection kit and detection method
A technology for detecting kits and procalcitonin, which is applied in biological testing, measuring devices, material inspection products, etc., can solve problems such as unstable test results, poor quantitative accuracy, and low sensitivity, and achieve clear clinical guidance and response Fast, sensitive results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
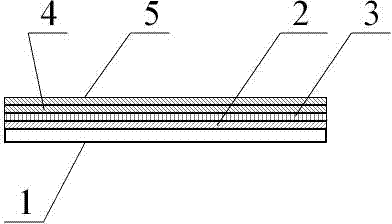
[0033] The various components of the test paper card in the procalcitonin detection kit can be prepared by the following measures:
[0034] 1. Preparation of sample pad 2:
[0035] Soak the glass fiber membrane in the treatment solution containing 2.0% Triton X-100, 3% BSA, 0.2M Tris buffer, pH7.5, soak at 4°C for 4 hours, then place it in an oven, and dry it at 37°C 2 hours.
[0036] 2. Preparation of binding pad 3 for absorbing fluorescent microsphere-labeled antibody:
[0037] Soak the glass fiber membrane in 200mM Tris-HCL treatment solution (containing 2.0% Triton X-100, 3.0% BSA, pH7.5), soak at 4°C for 4 hours, then take it out of the oven at 37°C and dry it for 4 hours, and set it aside. Put the glass fiber membrane on the Bio-DotXYZ3050 three-dimensional spraying platform, use the Bio-Jet Quanti300 non-contact micro-quantitative nozzle to spray the rare earth fluorescent microsphere-labeled procalcitonin monoclonal antibody on the glass fiber membrane, and dry i...
Embodiment 2
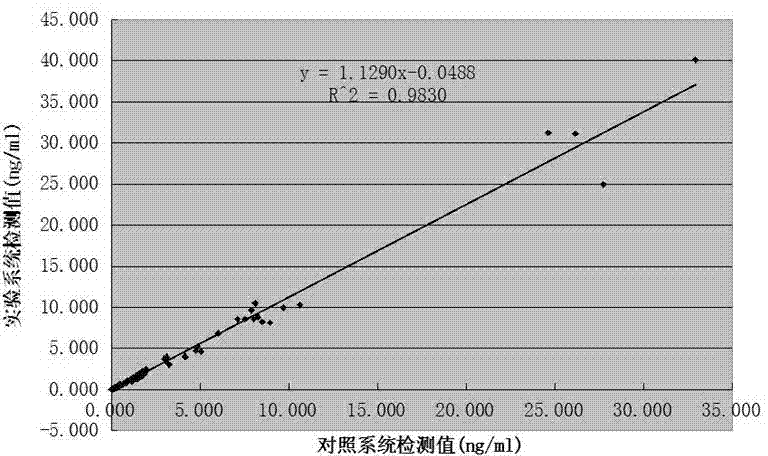
[0046] Example 2: accuracy test
[0047] Select the above test paper card and fluorescence immunochromatography analyzer (model: NEO-007),
[0048] Setting of the parameters of the fluorescence immunoassay analyzer: after setting the process parameters of the test paper card on the fluorescence immunoassay The original calcitonin calibrator is measured with a test paper card to obtain the fluorescence intensity value of each calibrator, and the result is input into the parameters of the analyzer to complete the setting of the parameters of the analyzer.
[0049] Main testing materials: clinical samples were obtained from relevant hospitals, a total of 200 electrochemiluminescence immunoassay value samples, including 100 serum samples, 100 whole blood samples, and the distribution range of procalcitonin content was between 0-40ng / mL.
[0050] Detection method:
[0051] Step 1: Equilibrate the detection reagent and sample to room temperature, take out the test paper card...
Embodiment 3
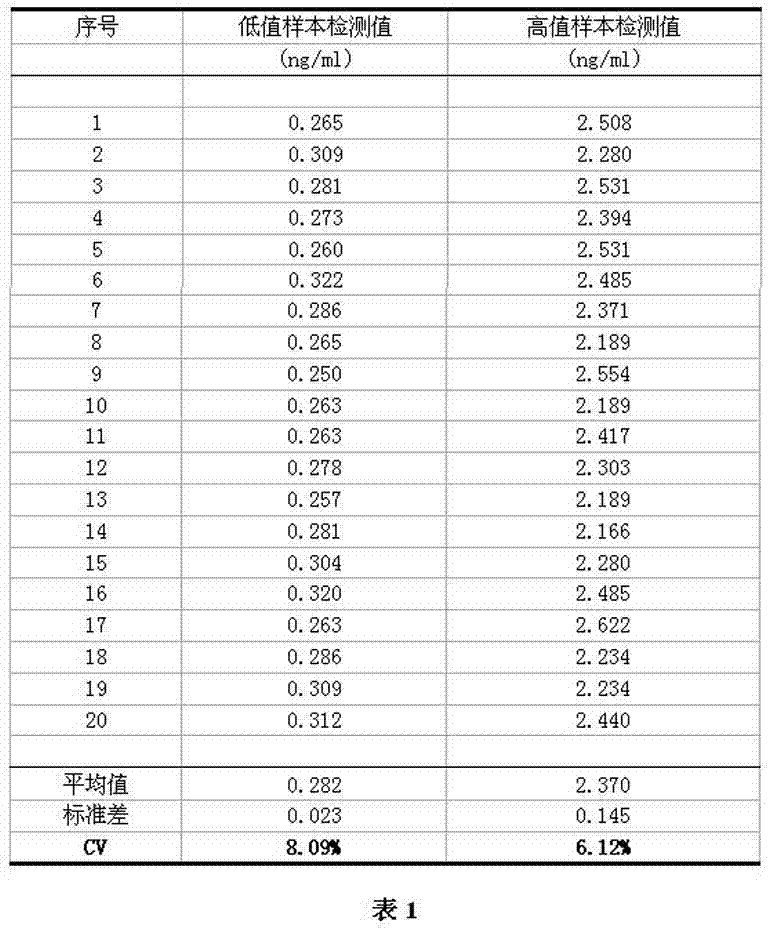
[0058] Example 3: precision test
[0059] Using the test paper card and measuring system of Example 2, the test paper card and the fluorescent immunochromatographic analyzer of the present invention were tested for precision.
[0060] Main testing materials: clinical samples obtained from relevant hospitals, a total of 2 serum samples with chemiluminescence immunoassay value, among which the clinical measurement value of the low value fixed value sample is 0.26ng / ml, and the clinical measurement value of the high value fixed value sample is 2.28ng / ml .
[0061] Detection method:
[0062] Using the test paper card and measuring system of Example 2, each of the 2 fixed-value samples was repeatedly measured 20 times.
[0063] Analysis of test results:
[0064] After the clinical sample test reagents are prepared, the clinical samples are tested according to the test method, and the test results are analyzed.
[0065] test results:
[0066] as attached image 3 As show...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com