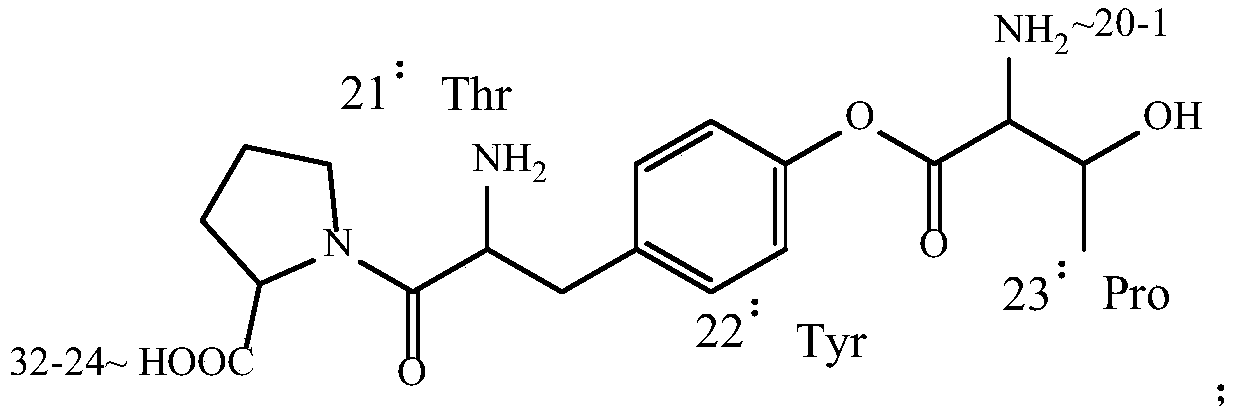
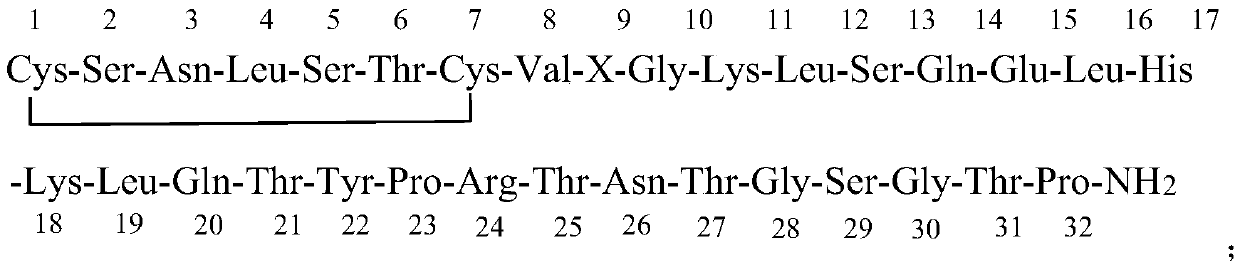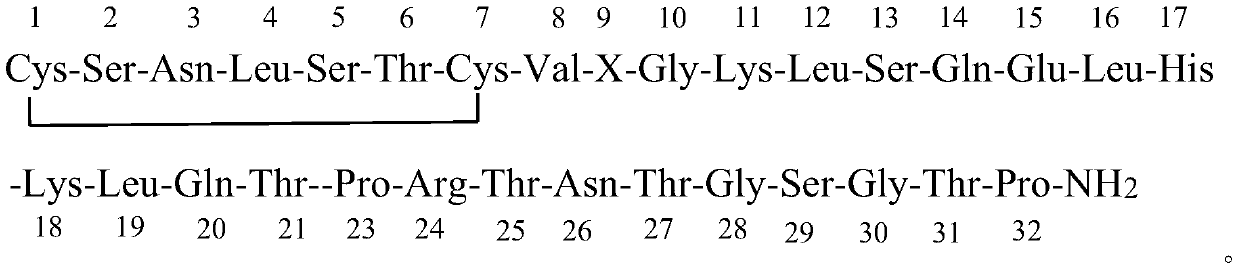Preparation method for salmon calcitonin, conjugated preparation of salmon calcitonin and use of conjugated preparation in drugs for osteoporosis
A technology for salmon calcitonin and osteoporosis, which is applied in the field of medicine, can solve the problems of incomplete protection, large impurities in salmon calcitonin, loss of salmon calcitonin, etc., and achieve simplified processing steps, high degree of condensation, and easy operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] A preparation method of salmon calcitonin, comprising the steps of:
[0040] The 23-32 position of the salmon calcitonin fragment prepared by the Fmoc-strategy solid-phase method:
[0041] -Pro-Arg(Pbf)-Thr(tBu)-Asn(Trt)-Thr(tBu)-Gly-Ser(tBu)-Gly-Thr(tBu)-Pro-Resin added Fmoc-Tyr(Alloc)-OH , DFIH, EEDQ and DMSO, reacted, dried with nitrogen, washed with DMSO, dried with nitrogen; added DMSO solution of hexahydropyridine, reacted for 15-25 minutes at 15-25°C, dried with nitrogen, washed with DMSO, dried with nitrogen to obtain -Tyr(Alloc)-Pro-Arg(Pbf)-Thr(tBu)-Asn(Trt)-Thr(tBu)-Gly-Ser(tBu)-Gly-Thr(tBu)-Pro-resin, where DFIH and Fmoc The molar ratio of -Tyr(Alloc)-OH is 1-1.5; the molar ratio of EEDQ to Fmoc-Tyr(Alloc)-OH is 0.1-0.5.
[0042] A preparation method of salmon calcitonin, comprising the steps of:
[0043] The 18-32 position of the salmon calcitonin fragment prepared by the Fmoc-strategy solid-phase method:
[0044]-Lys(Boc)-Leu-Gln(Trt)-Thr(tBu)-Tyr(Allo...
Embodiment 1
[0156] The steps for the condensation of salmon calcitonin 22 and 23, 17 and 18 are as follows, and the synthesis of other fragments refers to Comparative Example 1.
[0157] (1) The 22nd and 23rd condensation operation steps of salmon calcitonin:
[0158] -Pro-Arg(Pbf)-Thr(tBu)-Asn(Trt)-Thr(tBu)-Gly-Ser(tBu)-Gly-Thr(tBu)-Pro-Resin added Fmoc-Tyr(Alloc)-OH (MW: 487.51, 74.4mmol) 36.3g, DFIH 23.4g (MW: 262.11, 89.3mmol), EEDQ 1.8g (MW: 247.29, 7.44mmol) and 250g DMSO, reacted, dried with nitrogen, washed three times with DMSO, dried with nitrogen Add 300g25% hexahydropyridine DMSO solution, react for 20 minutes at 20°C, dry with nitrogen, wash with DMSO three times, and dry with nitrogen to obtain -Tyr(Alloc)-Pro-Arg(Pbf)-Thr(tBu)-Asn( Trt)-Thr(tBu)-Gly-Ser(tBu)-Gly-Thr(tBu)-Pro-resin.
[0159] (2) Condensation steps of 17-position and 18-position of salmon calcitonin:
[0160] -Lys(Boc)-Leu-Gln(Trt)-Thr(tBu)-Tyr(Alloc)-Pro-Arg(Pbf)-Thr(tBu)-Asn(Trt)-Thr(tBu)-Gly-Ser(tBu )-...
Embodiment 2
[0162] The steps for the condensation of salmon calcitonin 22 and 23 are as follows, and the synthesis of other fragments refers to Comparative Example 1.
[0163] Calcitonin 22 and 23 condensation operation steps: -Pro-Arg(Pbf)-Thr(tBu)-Asn(Trt)-Thr(tBu)-Gly-Ser(tBu)-Gly-Thr(tBu)- Add Fmoc-Tyr(Alloc)-OH (MW: 487.51, 74.4mmol) 36.3g, DFIH 29.3g (MW: 262.11, 111.6mmol), EEDQ 9.2g (MW: 247.29, 37.2mmol) and 250gDMSO to Pro-resin, Reaction, dry with nitrogen, wash with DMSO three times, dry with nitrogen; add 300g of 25% hexahydropyridine in DMSO, react at 20°C for 20 minutes, dry with nitrogen, wash with DMSO three times, and dry with nitrogen to obtain -Tyr(Alloc)-Pro -Arg(Pbf)-Thr(tBu)-Asn(Trt)-Thr(tBu)-Gly-Ser(tBu)-Gly-Thr(tBu)-Pro-resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com