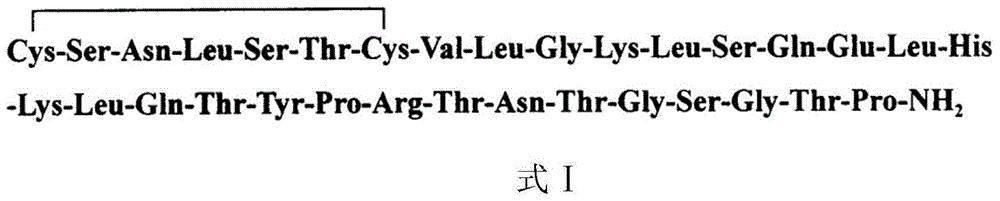Preparation method of salmon calcitonin acetate
A technology of salmon calcitonin and resin, which is applied to the preparation method of peptides, calcitonin, chemical instruments and methods, etc., can solve the problems of increased cost, large feeding, and difficult resin coupling of polypeptide fragments, so as to improve efficiency, The effect of reducing production costs and reducing effective utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Prepare salmon calcitonin peptide resin:
[0045] Using Fmoc-Pro-resin as the initial carrier, the salmon calcitonin peptide resin was prepared by de-Fmoc protection and coupling reaction, and the protected amino acids were sequentially coupled. Pro was used as the first coupled amino acid, and Cys was used as the last coupled amino acid. Amino acid sorting, coupled amino acid fragments are:
[0046] Fmoc-Cys(Trt)-Ser(tBu)-Asn(Trt)-X(tBu)-Thr(tBu)-Cys(Trt)-Val-Y-Lys(Boc)-X(tBu)-Gln(Trt) -Glu(OtBu)-Leu-His(Trt)-Lys(Boc)-Leu-Gln(Trt)-Thr(tBu)-Tyr(tBu)-Pro-Arg(Pbf)-Thr(tBu)-Asn(Trt )-Thr(tBu)-Z(tBu)-Thr(tBu)-Pro-resin,
[0047] Wherein X is Leu-Ser, Y is Leu-Gly, Z is Gly-Ser-Gly.
[0048] When X is inserted, the corresponding protected amino acid is Fmoc-Leu-Ser(tBu)-OH;
[0049] When Y is inserted, the corresponding protected amino acid is Fmoc-Leu-Gly-OH;
[0050] When Z is inserted, the corresponding protected amino acid is Fmoc-Gly-Ser(tBu)-Gly-OH.
[0051] Conc...
Embodiment 2
[0083] Prepare salmon calcitonin peptide resin:
[0084] Using Fmoc-Pro-resin as the starting carrier, the salmon calcitonin peptide resin was prepared by de-Fmoc protection and coupling reaction, and sequentially coupled the protected amino acids. Pro was used as the first coupled amino acid, and Cys was used as the last coupled amino acid. Amino acid sorting, coupled amino acid fragments are:
[0085] Fmoc-Cys(Trt)-Ser(tBu)-Asn(Trt)-X(tBu)-Thr(tBu)-Cys(Trt)-Val-Y-Lys(Boc)-X(tBu)-Gln(Trt) -Glu(OtBu)-Leu-His(Trt)-Lys(Boc)-Leu-Gln(Trt)-Thr(tBu)-Tyr(tBu)-Pro-Arg(Pbf)-Thr(tBu)-Asn(Trt )-Thr(tBu)-Z(tBu)-Thr(tBu)-Pro-resin,
[0086] Wherein X is Leu-Ser, Y is Leu-Gly, Z is Gly-Ser-Gly.
[0087] When X is inserted, the corresponding protected amino acid is Fmoc-Leu-Ser(tBu)-OH;
[0088] When Y is inserted, the corresponding protected amino acid is Fmoc-Leu-Gly-OH;
[0089] When Z is inserted, the corresponding protected amino acid is Fmoc-Gly-Ser(tBu)-Gly-OH.
[0090] Concrete...
Embodiment 3
[0111] Example 3: Adding amino acids one by one
[0112] (1), using RinkAmideMBHA resin or RinkAmideAM resin as the starting material, using Fmoc-protected amino acids as monomers, using TBTU / HOBt or HBTU / HOBt as condensing agents, connecting amino acids one by one to obtain protected thirty-docosopeptide resins, during which Remove the Fmoc-protecting group in turn; the reduced crude product is obtained;
[0113] (2), adding the peptide cutting reagent TFA / EDT / H2O / TIS to cut the peptide, and remove the side chain protection group, then add ether to precipitate the crude product, and obtain the crude product of reduced salmon calcitonin;
[0114] (3), after oxidizing by iodine / methanol solution, and filtering, the crude product of oxidized salmon calcitonin is obtained;
[0115] (4), separation and purification are carried out in C18 column or C8 to obtain the target product.
[0116] For specific implementation parameters, please refer to Example 1 or CN1865283B. The total...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com