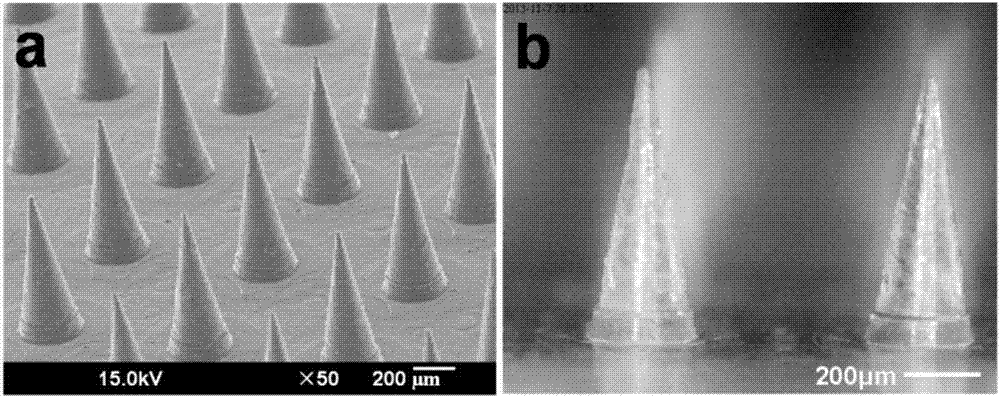
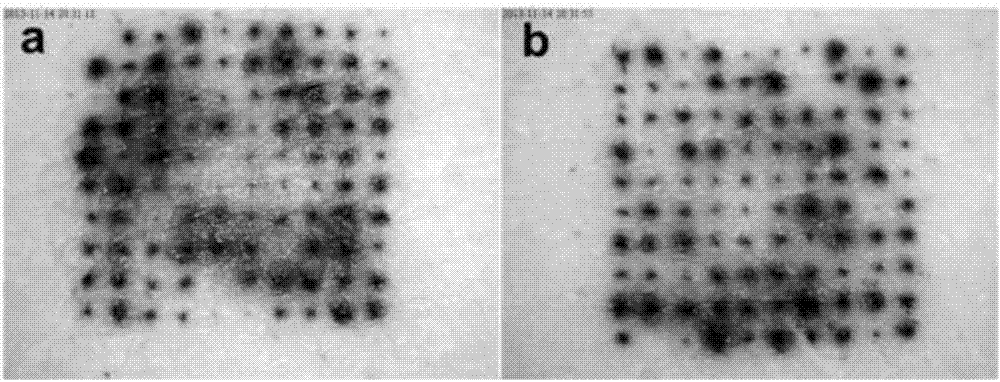
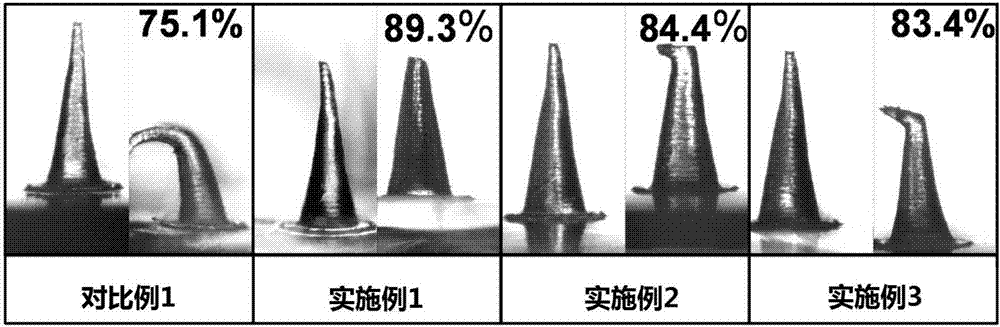
Salmon calcitonin soluble microneedle patch and preparation method thereof
A salmon calcitonin, soluble technology, applied in the directions of microneedle, needle, sheet delivery, etc., can solve the problem of no salmon calcitonin soluble microneedle research, etc., to achieve stability and large-scale production of microneedles , the effect of improving drug administration safety and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Preparation of Salmon Calcitonin Soluble Microneedle Patch
[0047] The preparation method of the salmon calcitonin soluble microneedle patch of the present embodiment is as follows:
[0048] (1) sCT / Dex-Tre microneedle preparation solution: Weigh 2.5mg sCT and dissolve it in 50μL deionized water, and take another 25mg dextran 40 (molecular weight is 40000), and 12.5mg D-trehalose dihydrate are dissolved in the above solution, Swell overnight to prepare sCT / Dex-Tre microneedle preparation solution.
[0049] (2) Blank base solution: Dissolve 50 mg of polyvinylpyrrolidone K90 (PVPK90, molecular weight: 1,100,000) in 150 μL of ethanol, stir evenly, and swell overnight to prepare blank base solution K90E.
[0050] (3) Microneedle patch preparation
[0051] Inject the sCT / Dex-Tre microneedle body preparation solution into the microneedle female mold, centrifuge for 10 minutes under the force of 3000g, and the centrifugation temperature is 4°C. After the solution fi...
Embodiment 2
[0057] The preparation method of the salmon calcitonin soluble microneedle patch of the present embodiment is as follows:
[0058] (1) sCT / Dex-Tre microneedle preparation solution: Weigh 2.7mg sCT and dissolve in 50μL deionized water, and take another 24.3mg dextran 40 (molecular weight 40000), 10.8mg D-trehalose dihydrate and dissolve in the above solution , and swell overnight to prepare the sCT / Dex-Tre microneedle preparation solution.
[0059] Other steps are the same as in Example 1.
Embodiment 3
[0061] The preparation method of the salmon calcitonin soluble microneedle patch of the present embodiment is as follows:
[0062] (1) sCT / Dex-Tre microneedle preparation solution: Weigh 2.3mg sCT and dissolve it in 50μL deionized water, and take another 25.3mg dextran 40 (molecular weight 40000), 13.8mg D-trehalose dihydrate and dissolve it in the above solution , and swell overnight to prepare the sCT / Dex-Tre microneedle preparation solution.
[0063] Other steps are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
| Bottom diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com