PCT (procalcitonin) and CRP quantitative joint inspection chromatography test strip and preparation method thereof
An immunochromatographic test strip and detection line technology, applied in the field of medical testing, can solve the problems of lack of quantitative joint inspection of PCT and CRP, low sensitivity, immunotransmission turbidimetric methodology and clinical application need further verification, etc. Reduce the risk of errors, reduce costs, and reduce usage effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 PCT and CRP quantitative combined detection immunochromatographic test strip and preparation method thereof
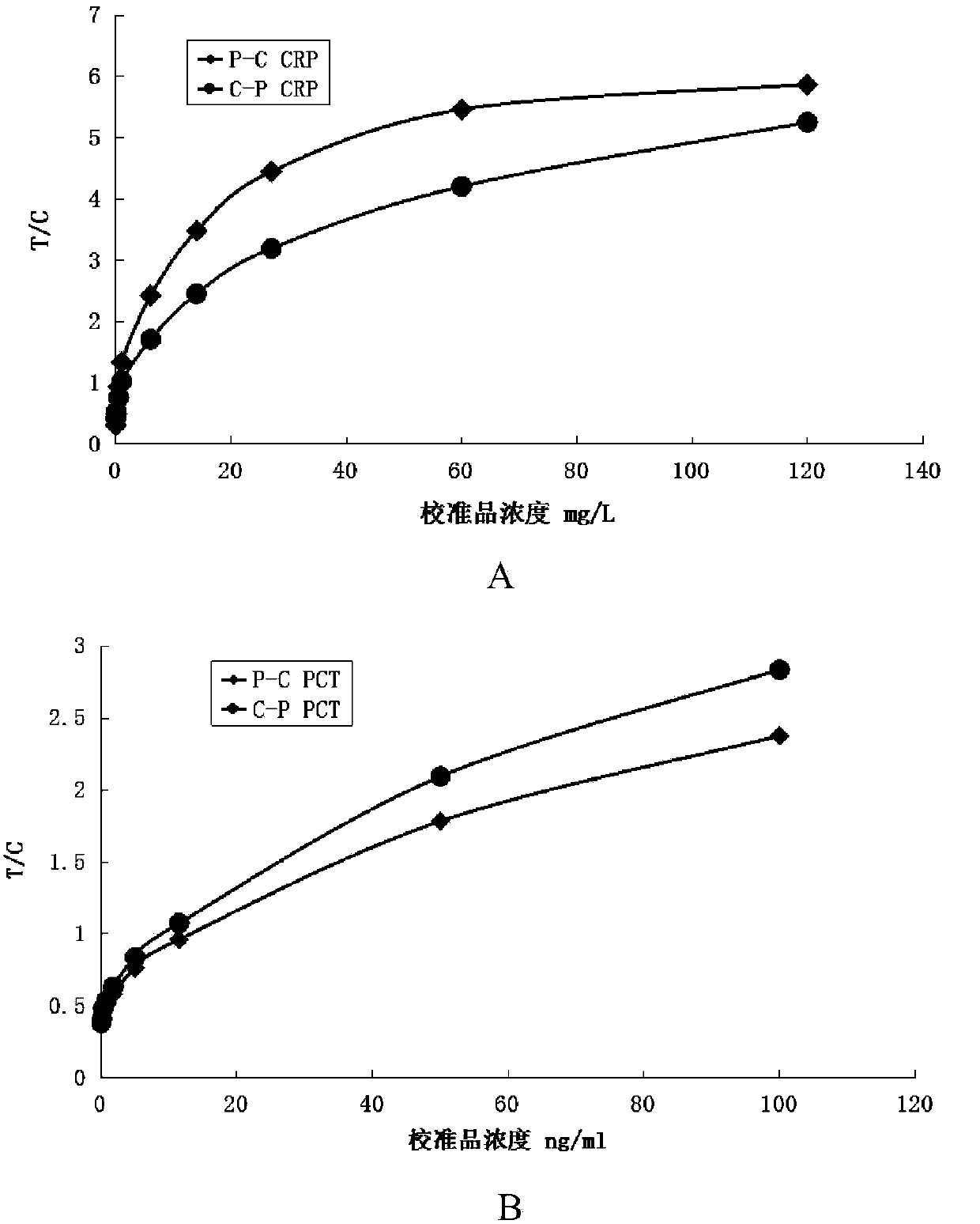
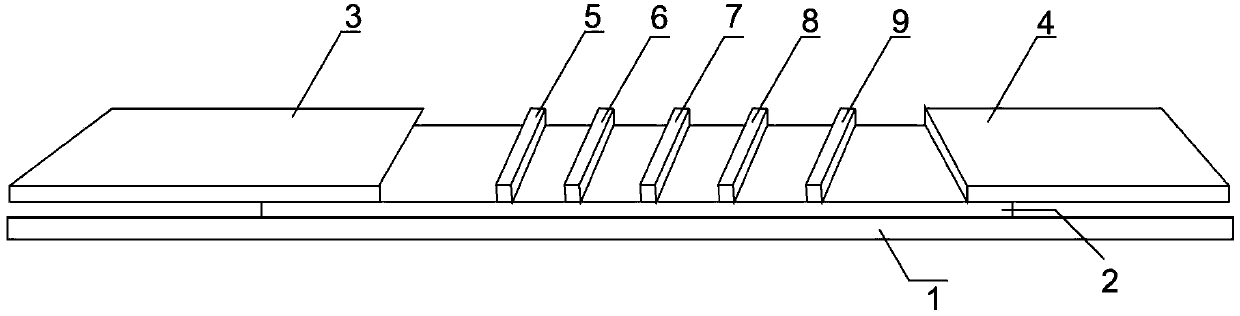
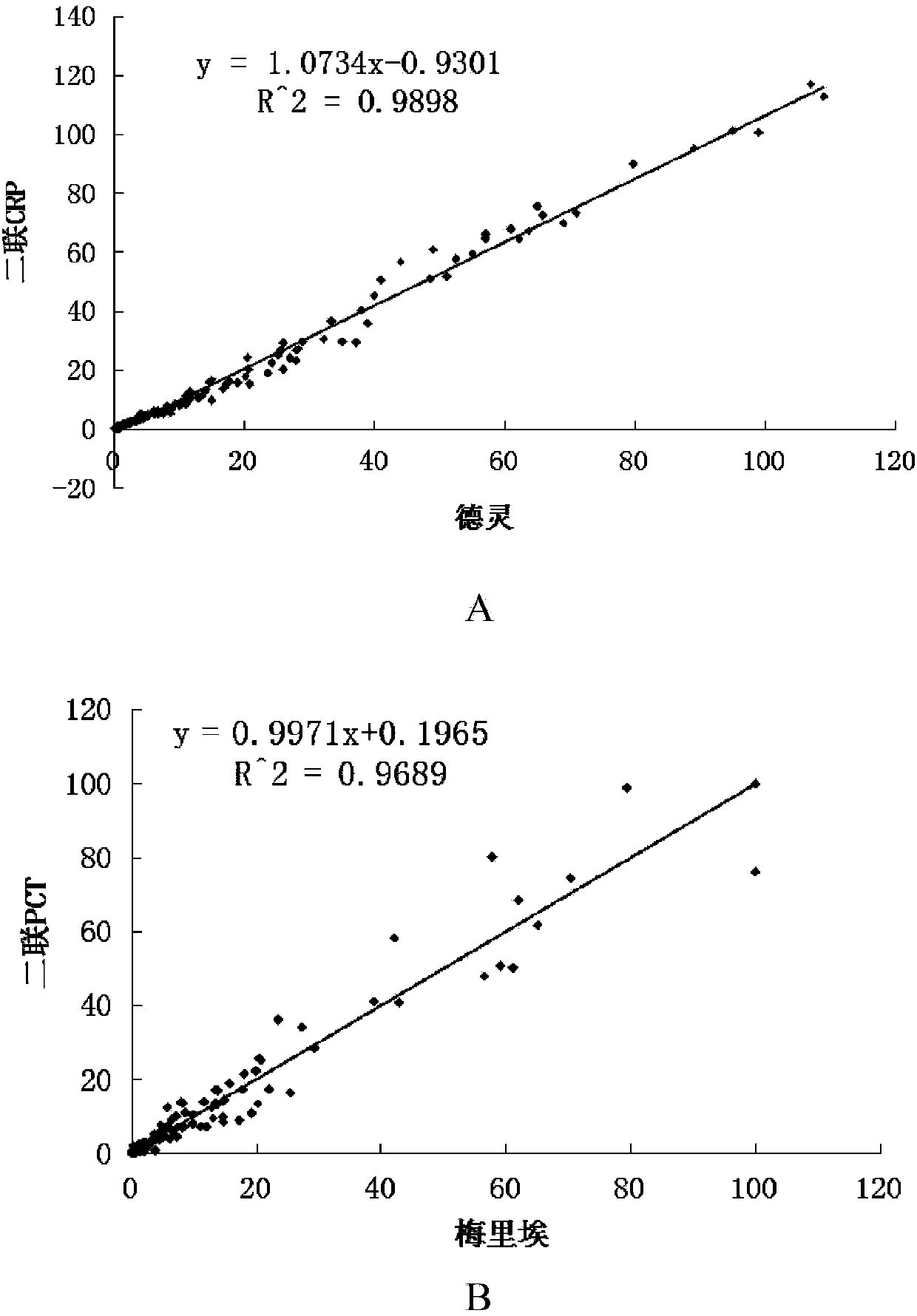
[0058] Such as figure 1 Shown is a structural schematic diagram of the PCT and CRP quantitative combined detection immunochromatographic test strip of the present invention. A PCT and CRP quantitative combined detection immunochromatographic test strip, comprising a substrate 1 and a coating film 2 arranged on the substrate 1, the coating film 2 is provided with a sample pad 3, a coating line area and Absorbent paper 4, the coated line area includes the fluorescent latex coated with CRP monoclonal antibody, PCT monoclonal antibody and goat antibody, which are arranged in parallel in turn near the 3rd end of the sample pad to the 4th end of the absorbent paper and are spaced apart from each other. The labeling line 5 of the chicken IgY antibody, the quality control line 6 coated with the chicken IgY antibody, the CRP detection line 7 coated with anothe...
Embodiment 2
[0074] Example 2 PCT and CRP quantitative combined detection immunochromatographic test strip and preparation method thereof
[0075] The structure of the test strip of this embodiment is the same as that of the test strip of Example 1. The difference is that in this embodiment, the coating membrane 2 is a nitrocellulose membrane with a climbing speed of 110 s / 4cm.
[0076] The preparation method of the PCT and CRP quantitative joint detection immunochromatography test strip of this embodiment is as follows, comprising the following steps:
[0077] 1. Covalent Activation of Fluorescent Latex
[0078] With embodiment 1.
[0079] 2. Preparation of Fluorescent Latex Microparticle-labeled Proteins
[0080] With embodiment 1.
[0081] 3. Preparation of Nitrocellulose Coated Membrane
[0082] a. Preparation of marker lines
[0083] Dilute the fluorescent latex labeled with CRP monoclonal antibody, the fluorescent latex labeled with PCT monoclonal antibody and the fluorescent l...
Embodiment 3
[0092] Example 3 PCT and CRP quantitative combined detection immunochromatographic test strip and preparation method thereof
[0093] The structure of the test strip of this embodiment is the same as that of the test strip of Example 1. The difference is that in this embodiment, the coating membrane 2 is a nitrocellulose membrane with a climbing speed of 135 s / 4cm.
[0094] The preparation method of the PCT and CRP quantitative joint detection immunochromatography test strip of this embodiment is as follows, comprising the following steps:
[0095] 1. Covalent Activation of Fluorescent Latex
[0096] With embodiment 1.
[0097] 2. Preparation of Fluorescent Latex Microparticle-labeled Proteins
[0098] With embodiment 1.
[0099] 3. Preparation of Nitrocellulose Coated Membrane
[0100] a. Preparation of marker lines
[0101] Dilute the fluorescent latex labeled with CRP monoclonal antibody, the fluorescent latex labeled with PCT monoclonal antibody and the fluorescent l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com