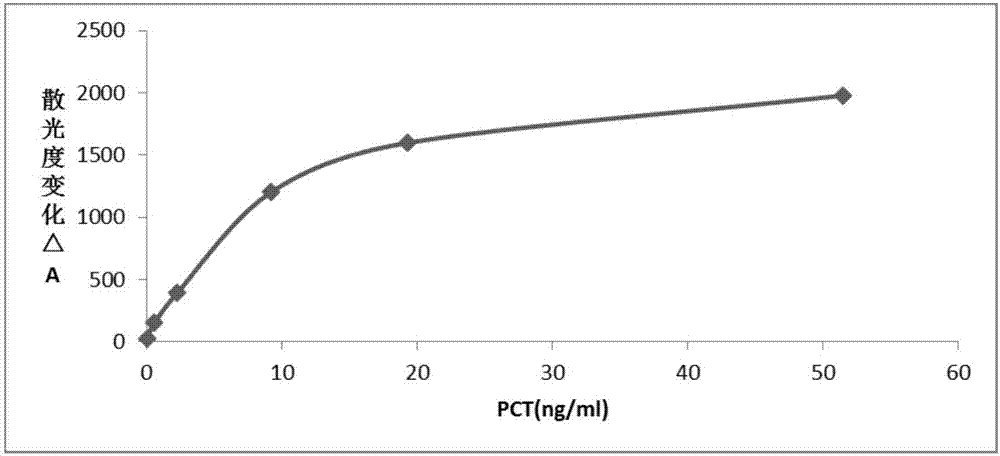
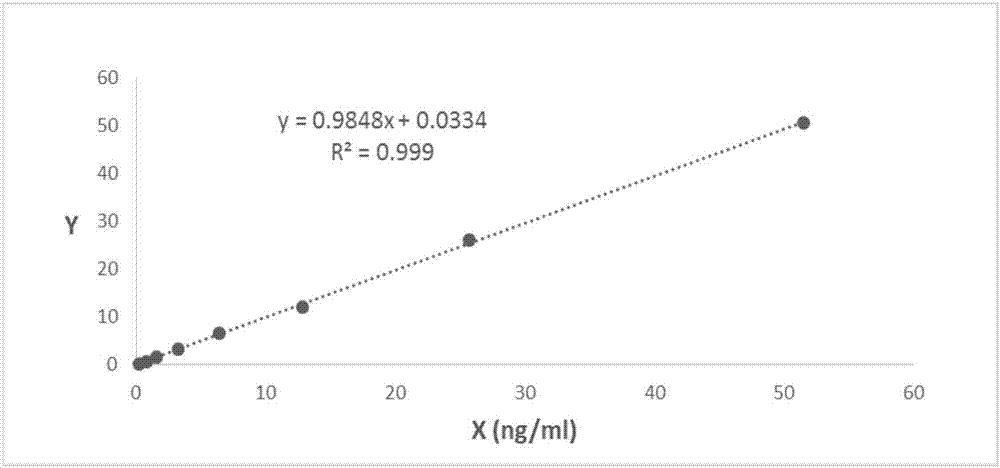
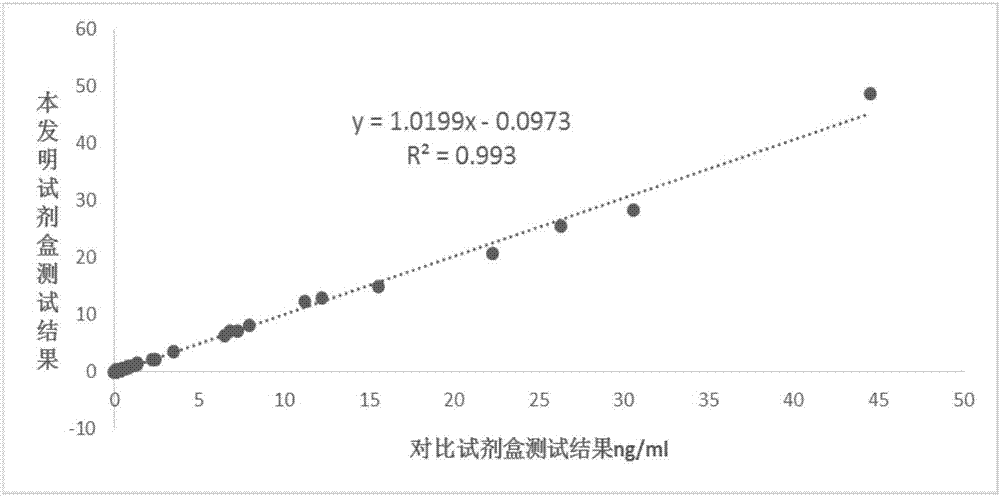
Immunoturbidimetric kit for detecting procalcitonin
A procalcitonin and kit technology, applied in the field of medical immunization, can solve the problems of complicated operation, narrow linear range, and increase the pain of subjects, and achieve the effects of high detection sensitivity, broadened linear range, and wide linear range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 kit of the present invention
[0034] 1) Preparation of reagent R1:
[0035] Weigh 24.2g Trizma-base, 7.9g NaCL, 10g BSA, 10g PEG6000, 3g SDS, 5g Tween 80, 1g EDTA and 1g sodium azide according to the weight of the formula and dissolve them in 0.8L distilled water, adjust the pH value to 8.0, set Fill to the 1L scale line, filter to get R1;
[0036] 2) Preparation of reagent R2:
[0037] Step 1: Combine carboxylated polystyrene latex microspheres with particle sizes of 80nm and 198nm in a certain proportion, wash with distilled water 3 times, add 200mmol / L PBS buffer to dilute the polystyrene latex microspheres to 0.01mg / ml;
[0038] Step 2: Add 4 mg EDC and 2 mg NHS to 10 ml of diluted latex microspheres, stir continuously at room temperature for 30 minutes, then centrifuge, wash with PBS buffer to remove unreacted EDC and NHS, and dilute to 0.01 mg / ml;
[0039] Step 3: Dissolving and diluting the PCT antibody to 1 mg / ml with Tris buffer solution of Ph8...
Embodiment 2
[0041] Embodiment 2 kit of the present invention
[0042] 1) Preparation of reagent R1:
[0043] Weigh 12.1g Trizma-base, 15g NaCL, 10g BSA, 20g PEG6000, 5g SDS, 3g Tween 80, 1g EDTA and 1g sodium azide in 0.8L distilled water, adjust the pH value to 8.0, and constant volume To the 1L scale line, filter to get R1;
[0044] 2) Preparation of reagent R2:
[0045] Step 1: Combine carboxylated polystyrene latex microspheres with particle sizes of 123nm and 220nm in a certain proportion, wash with distilled water for 3 times, add 100mmol / L PBS buffer to dilute the polystyrene latex microspheres to 0.01mg / ml;
[0046] Step 2: Add 2 mg EDC and 4 mg NHS to 10 ml of diluted latex microspheres, stir continuously at room temperature for 30 minutes, then centrifuge, wash with PBS buffer to remove unreacted EDC and NHS, and dilute to 0.01 mg / ml;
[0047] Step 3: Dissolving and diluting the PCT antibody to 1 mg / ml with Tris buffer solution of Ph8.0, then adding the PCT antibody diluent...
Embodiment 3
[0049] Embodiment 3 kit of the present invention
[0050] 1) Preparation of reagent R1:
[0051] Weigh 23.83g Hepes, 20g NaCL, 30g BSA, 30g PEG6000, 5g dodecyltrimethylammonium chloride, 3g Tween 80, 1g EDTA and 1g sodium azide according to the formula quality and dissolve them in 0.8L distilled water, adjust When the pH value reaches 8.0, set the volume to the 1L mark, and filter to obtain R1;
[0052] 2) Preparation of reagent R2:
[0053] Step 1: Combine carboxylated polystyrene latex microspheres with particle sizes of 107nm and 309nm in a certain proportion, wash with distilled water for 3 times, add 100mmol / L MES buffer to dilute the polystyrene latex microspheres to 0.01mg / ml;
[0054] Step 2: Add 2mgEDC and 4mgNHS to 10ml of diluted latex microspheres, stir continuously at room temperature for 30 minutes, then centrifuge, wash with 200mmol / LPBS buffer to remove unreacted EDC and NHS, and dilute to 0.01mg / ml;
[0055] Step 3: Dissolve and dilute the PCT antibody ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com