Anti-Blys monoclonal antibody and pharmaceutical composition containing anti-Blys monoclonal antibody
A monoclonal antibody, antibody technology, used in drug combinations, antibodies, anti-tumor drugs, etc., can solve problems such as high patient costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Preparation of hybridoma cells expressing anti-human Blys monoclonal antibody
[0068] Production of murine monoclonal antibodies by hybridoma cell technology. For the experimental protocol, please refer to the literature (Ed Harlow, David Lane. Antibody: A laboratory manual. 1988).
[0069] Firstly, Blys-His (containing 6×his tag) fusion protein is prepared by recombinant technology. The DNA sequence of Blys-his was cloned into PCDNA3.1 (Invitrogen), and the plasmid was transfected into CHO-K1 (ATCC No.CCL-60) cell line, and the stable expression of Blys-His was obtained through G418 (GIBCO) pressure selection Cell lines were cultured in serum-free medium, the culture supernatant was collected, and the expressed Blys-His fusion protein was purified with NI column (QIAGEN).
[0070] BALB / c mice were first immunized with purified Blys-His (as an antigen component) mixed with complete Freund's adjuvant (Sigma) for intraperitoneal injection, and then, on the 1...
Embodiment 2
[0073] Example 2: Preparation and identification of mouse monoclonal antibody against human Blys
[0074] The hybridoma cells were cultured in serum-free medium, the culture supernatant was collected, and the mouse monoclonal antibody was purified with Protein G column (GenScript) to obtain the monoclonal antibody called H1104-30. Antibody identification items are as follows:
[0075] A: Antibody binding activity
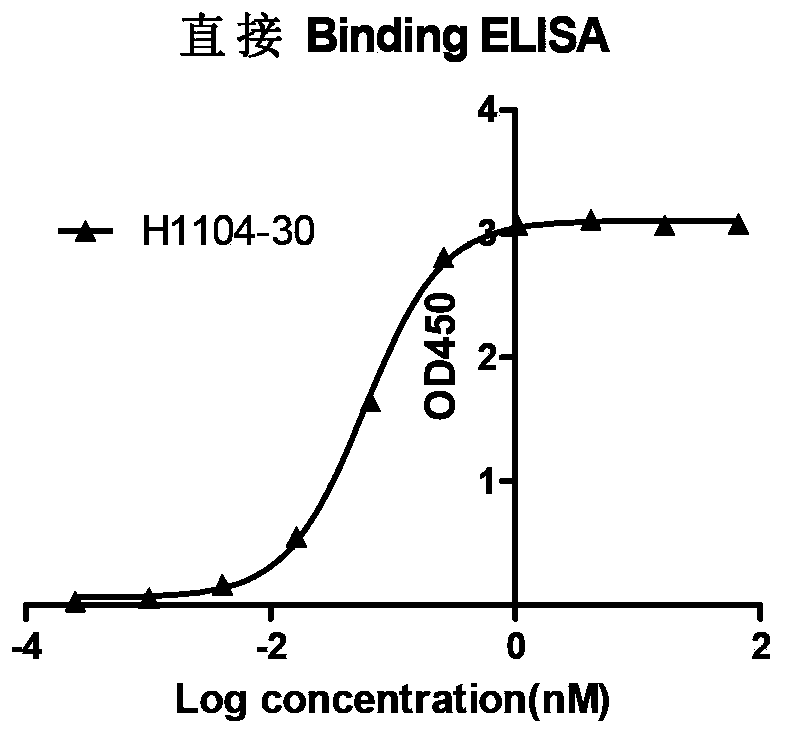
[0076] Coat Blys-His on a 96-well ELISA plate (Costar), then add 1:4 serially diluted monoclonal antibody to the wells of the coated Blys-His plate, add goat anti-mouse IgG antibody-HRP (Jackson Immuno), chromogenic for detection. The results showed that the screened monoclonal antibodies directly Binding EC 50 up to 10-100pM levels (see figure 1 ).
[0077] B: Binding specificity of antibody
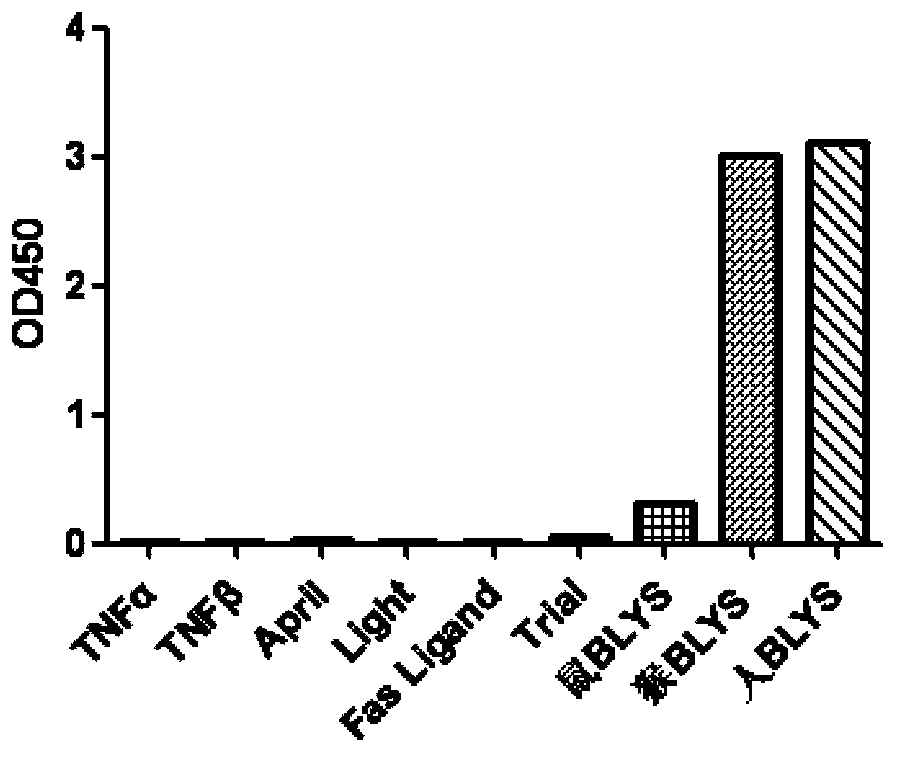
[0078] Add equal amounts of Blys-His, other TNF family members, including TNFα (R&D), TNFβ (R&D), April (R&D), Light (R&D), Fas Ligand (R&D), Trial (R&D), and mouse BLY...
Embodiment 3
[0083] Example 3: In Vitro Activity Determination of Monoclonal Antibodies
[0084] Blys antibodies inhibit the Blys signaling pathway by binding to Blys and blocking its binding to receptors. The in vitro activities include that the antibody inhibits the binding of Blys to the receptor and inhibits the proliferation of mouse splenocytes stimulated by Blys.
[0085] A: The antibody inhibits the binding activity of Blys to the receptor
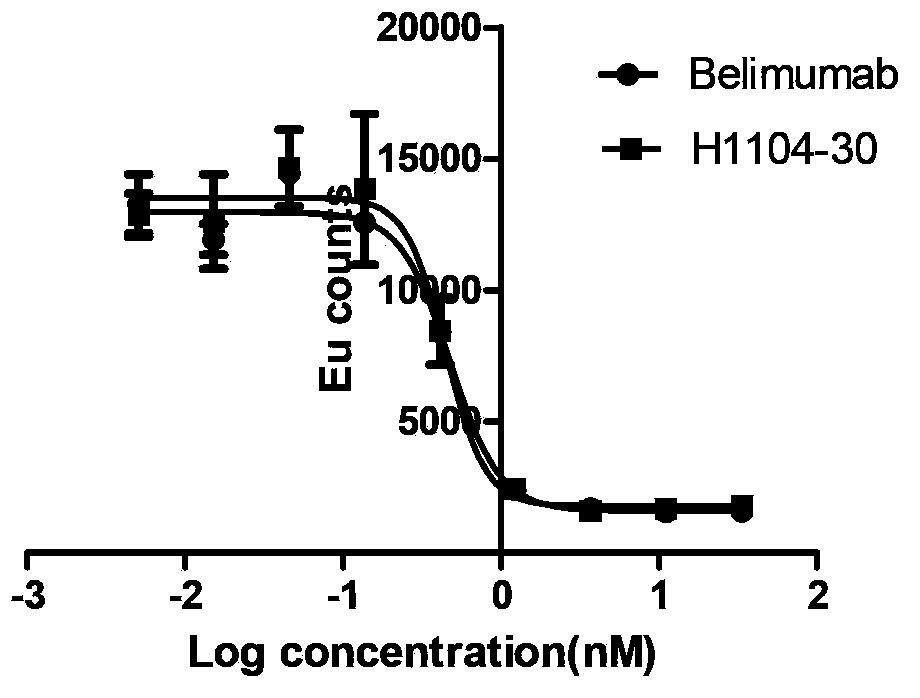
[0086]IM9 cells (ATCC No.CCL-159) (Blys receptor overexpression) were fixed on Poly-lysine 96-well plate (NUNC), 1:3 serially diluted antibody and biotinylated Blys were co-incubated for 1h, and then PBS Wash 3 times and detect with fluorescently labeled streptavidin (Perkin Elmer) (refer to Kevin P. Baker, 1 Bryan M. Edwards. Generation and Characterization of LymphoStat-B, a Human Monoclonal Antibody That Antagonizes the Bioactivities of B Lymphocyte Stimulator. ARTHRITIS & RHEUMATISM. Vol. 48, No. 11, November 2003, pp3253–3265). The resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com