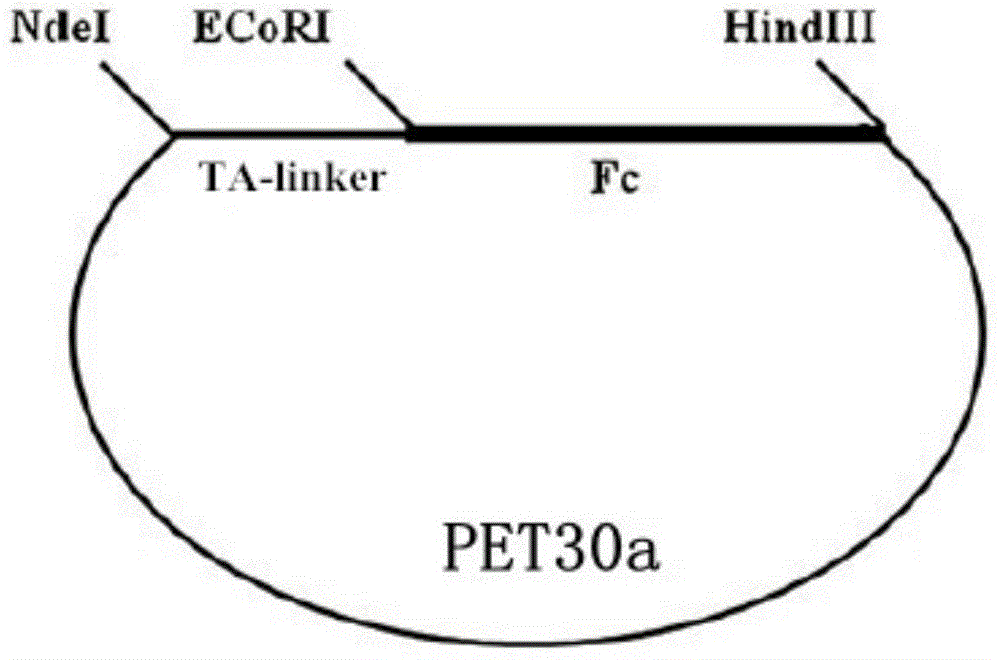
blys antagonistic peptide, plasmid containing ta-fc fusion protein gene and ta-fc fusion protein
A fusion protein and antagonistic peptide technology, applied in the field of biomedicine, can solve problems such as short half-life, low efficiency, and unstable peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Structural information on the interaction between BLyS and its receptors and the design of novel antagonistic peptides targeting BLyS
[0042] The interaction mode between BLyS and its receptor TACI was explored by computer-aided molecular docking and dynamic simulation, and the key amino acid residues for mutual recognition between BLyS and its receptor were analyzed by means of computer graphics and distance geometry.
[0043] Using computer-aided molecular design and high-throughput virtual screening technology to de novo design short peptides that can simulate the conformation of key amino acids in receptors, and then theoretically evaluate the role of BLyS and antagonistic peptides by means of de novo construction, molecular docking, and kinetic simulation; use computer graphics Biochemical techniques, distance geometry, and intermolecular hydrogen bonding interactions were used to evaluate potential biological effects of antagonistic peptides. After theoretical sc...
Embodiment 2
[0045] Chemical Synthesis of BLyS Antagonistic Peptide
[0046] According to the results of computer-aided design. We commissioned a biological company to prepare BLyS antagonistic peptide (TA), which has a purity of over 95%.
[0047] SampleId: TA, Sequence: DTSKLASTGYSSDPY (SEQ ID NO.1), MolecularWeight: 1591.66, HPLCAnalysis: PeptidePurity>95%.
Embodiment 3
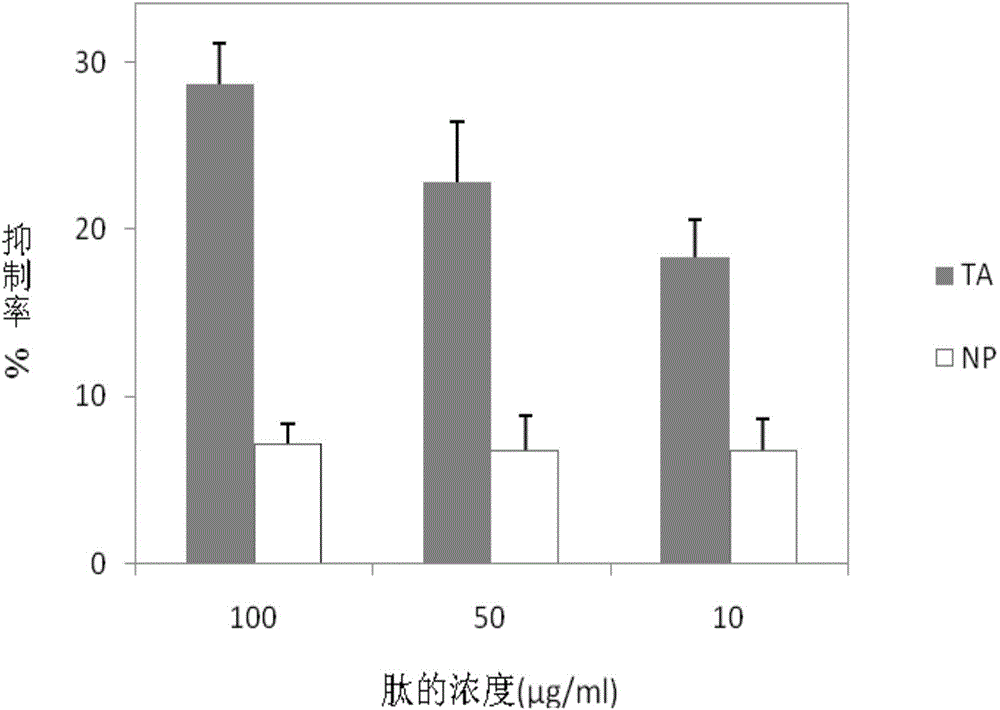
[0049] ELISA experiment to verify the inhibitory activity of antagonistic peptide TA
[0050] Dilute BLyS protein with coating solution to a final concentration of 10 μg / ml, take 50 μL in a microtiter plate, 4°C, 18h. Wash the microtiter plate 3 times with PBST, 5 min each time. Add 150 μl of 5% skimmed milk powder to each well, and incubate at 37°C for 2 hours. Wash the microtiter plate 3 times with PBST, 5 min each time. Mix a fixed concentration of TACI-Fc protein (final concentration of 10 μg / ml) with different concentration gradients of antagonistic peptide TA (final concentrations of 0, 10, 50 and 100 μg / ml), and each concentration has 3 parallels, each well Add 50 μL, and incubate at 37°C for 1h. The irrelevant peptide NP served as a control. Wash the microtiter plate 3 times with PBST, 5 min each time. Add 50 μL of HRP-labeled goat anti-human antibody diluted 1:5000 times to each well, 37°C, 1h. Wash 3 times with PBST, 5min each time. Add 50 μL TMB chromogenic s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com