Preparation of a quality control reference strain for lymphoblastic leukemia fusion gene detection
A technology that combines genes and leukemia, applied in the medical field, can solve problems that affect detection methods, cannot be repeated, and have a high false positive rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
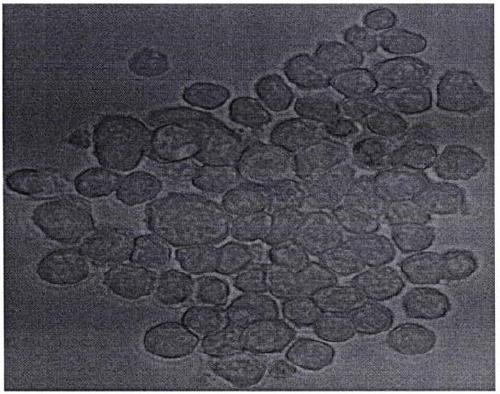
[0013] figure 1 It is a hybrid cell clone diagram prepared by the present invention.
[0014] exist figure 1 In the medium, the bone marrow cells of patients with lymphoid leukemia are induced by B lymphocyte stimulating factors to differentiate lymphocytes of various stages. Under the action of PEG, after fusion with mouse myeloma cells, they are screened by HAT for 1 to 2 weeks, and then viewed under an inverted microscope. Shoot (40X) to obtain a hybrid cell clone map.
[0015] Combine below figure 1 , the embodiment of the present invention is described in detail.
[0016] 1. Cells to be fused
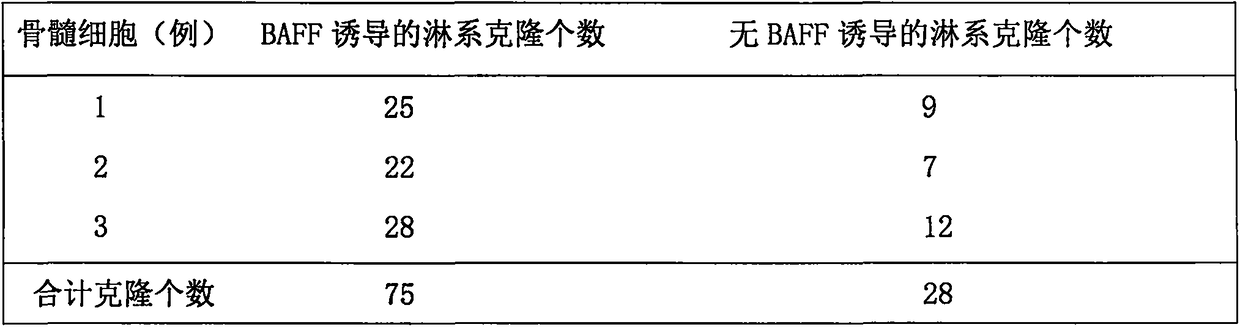
[0017] (1) Bone marrow cells to be fused: Bone marrow cells from patients with lymphocytic leukemia who were diagnosed and left after bone marrow cytology examination, containing children's EL / AML1 (46%), E2A / PBX1 (20%), Fusion genes such as BCR / ABLp190, BCR / ABLp210 and / or MLL / AF4, or fusion genes such as M-bcr / abl and / or m-bcr / abl in adults.
[0018] (2) Myeloma cells: mouse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com