Complex whorl aryl fluorene material, preparation and application method thereof
A technology of spiroaryl fluorene and its application method, which is applied in the field of complex spiroaryl fluorene materials and its preparation, can solve problems such as emission spectrum narrowing, and achieve high thermal stability and high glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The preparation method of complex spiroaryl fluorene compound material I is prepared by Friedel-Crafts reaction and Suzuki reaction catalyzed by boron trifluoride etherate complex. Taking compound material II as an example, the reaction is as follows:
[0046]
[0047] First, prepare the tertiary alcohol tripolyfluorene structural unit, by step (1) Suzuki coupling reaction, it is the carbon-carbon bond coupling reaction that palladium catalyzes alkylfluorene boronic acid and dibromofluorene tertiary alcohol; The consumption of catalyst is 0.1 to 20mol%; the suitable solvent is a weak polar or polar aprotic organic solvent or its mixed solvent; the reaction temperature is 30 to 150°C; the reaction time is 1 to 7 days. Then, bis(biphenyl)spirothiophene fluorene was prepared by the corresponding Suzuki reaction in step (2). Finally, the compound material II is obtained by the Friedel-Crafts reaction in step (3), and the specific reaction conditions are that bis(biphenyl...
Embodiment 1
[0049] Example 1, preparation of complex spiroaryl fluorene compound material II:
[0050] 2,7-bis(9,9-dioctyl-fluoren-2-yl)-9-phenyl-fluoren-9-ol
[0051]
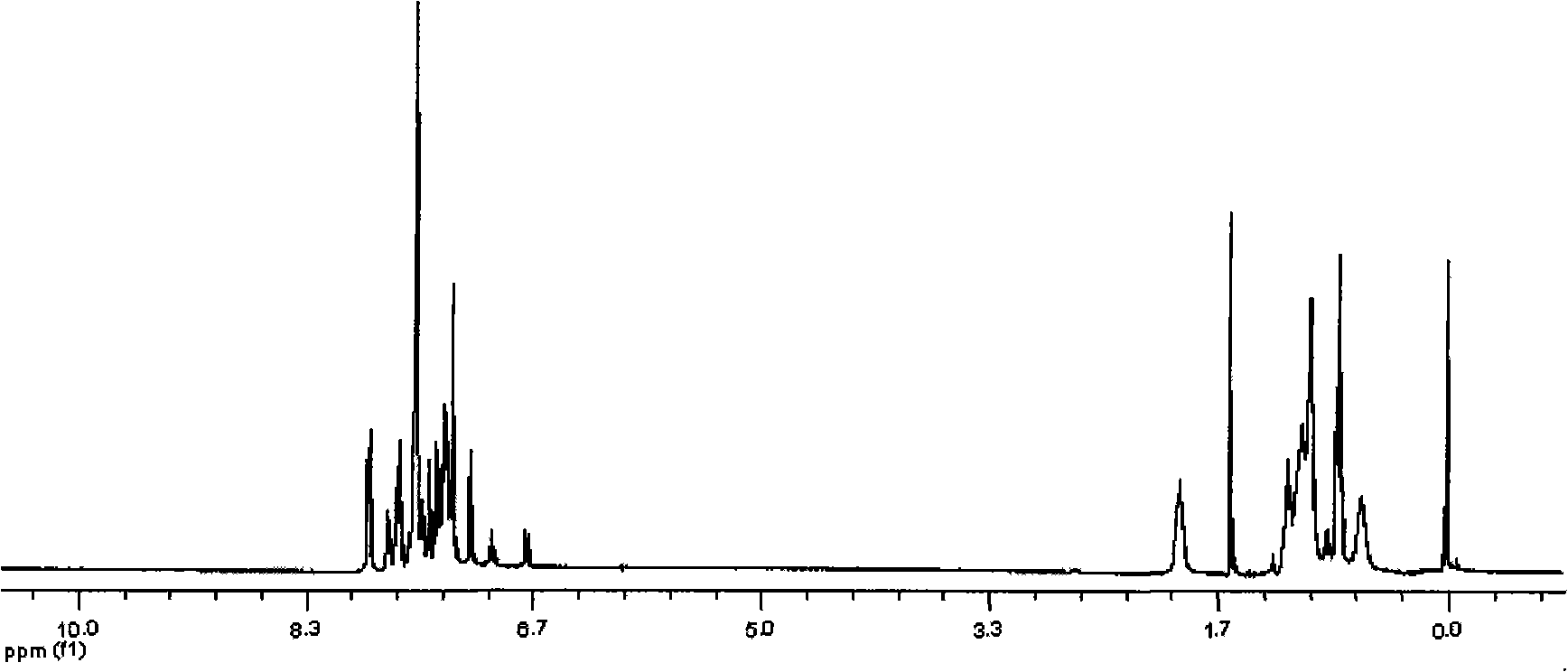
[0052] Take 2,7-dibromo-9-phenyl-fluoren-9-ol (1.735g, 4.17mmol, 1 equiv.) and 4,4,5,5-tetramethyl-2-(9,9-dioctyl Base-9H-fluoren-7-yl)-1,3,2-dioxabororane (4.524g, 8.75mmol, 2.1equiv.) was mixed and dissolved in solvent toluene (40mL), and catalyst Pd(PPh 3 ) 4 (50 mg, 0.043 mmol). Protect from light and pass through nitrogen, then add K 2 CO 3 (2.0M aqueous solution, 12.0mL, 24.0mmol), react at 90°C for 48 hours, add water after the reaction, use CHCl 3 Extraction, drying and rotary evaporation, petroleum ether silica gel column purification, to obtain solid 2,7-bis(9,9-dioctyl-fluoren-2-yl)-9-phenyl-fluoren-9-ol as colorless glass (3.28g, 76 %). MALDI-TOF-MS (m / z): 1034.7 (M + ), 1034.9. 1 H NMR (400MHz, CDCl 3 , ppm): δ7.815-7.795 (d, J=8.0Hz, 2H), 7.733-7.701 (t, 6H), 7.669 (s, 2H), 7.574-7.537 (m, 6H), ...
Embodiment 3
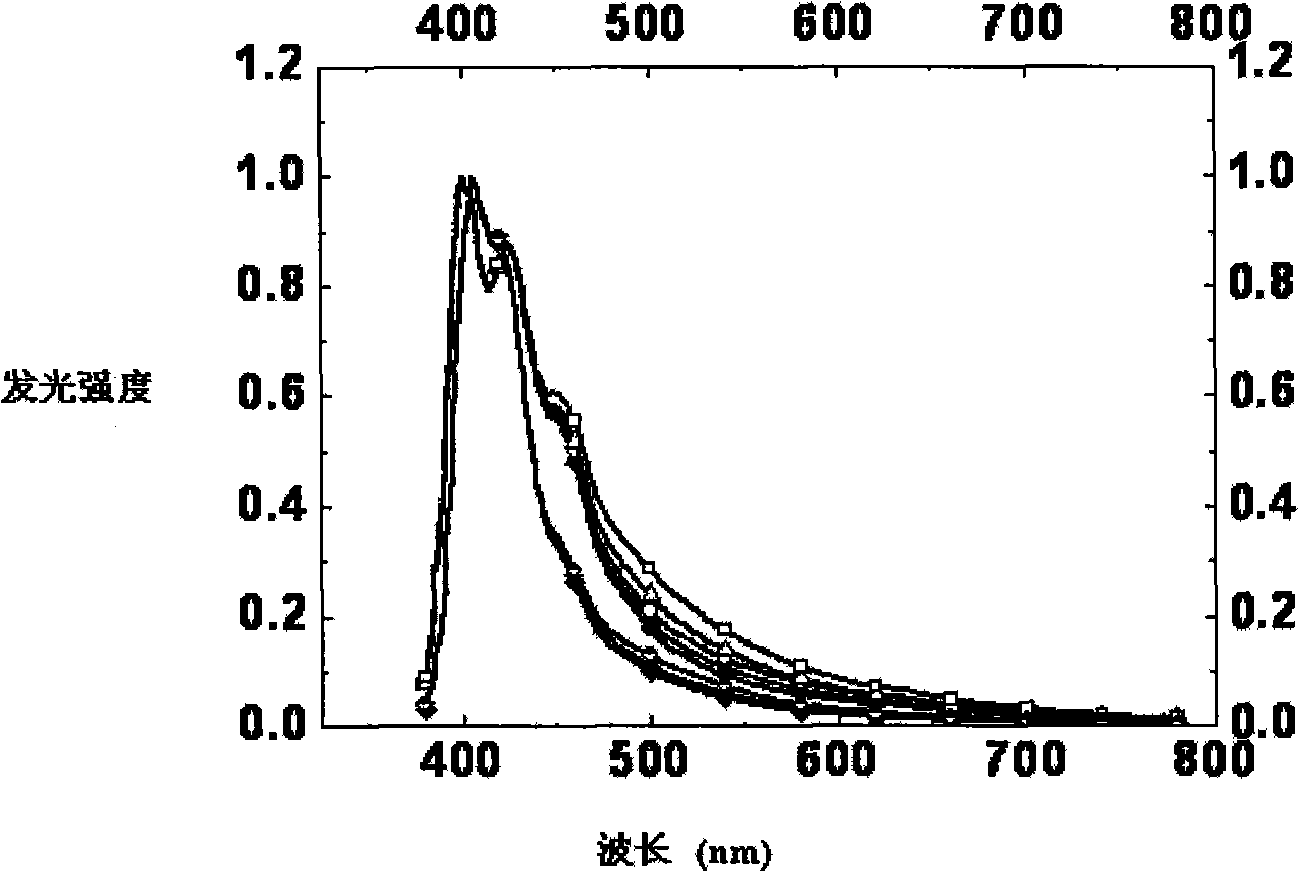
[0056] Embodiment 3, the photoluminescence spectrum measurement of complex spiroaryl fluorene material:
[0057] The product was made into a precise 1 μM dilute solution in chloroform and flushed with argon to remove oxygen. Shimadzu UV-3150 ultraviolet-visible spectrometer and RF-530XPC fluorescence spectrometer were used to measure the absorption spectrum and emission spectrum, and the photoluminescence spectrum was measured at the maximum absorption wavelength of ultraviolet absorption. The solid film was prepared by solution spin-coating film forming technology, and the film thickness was 300nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com