1,8-carbazoles polymer photovoltaic material, preparation and use method thereof
A technology of optoelectronic materials and polymers, applied in the fields of luminescent materials, chemical instruments and methods, photovoltaic power generation, etc., can solve the problem of no patent reports on polymer materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
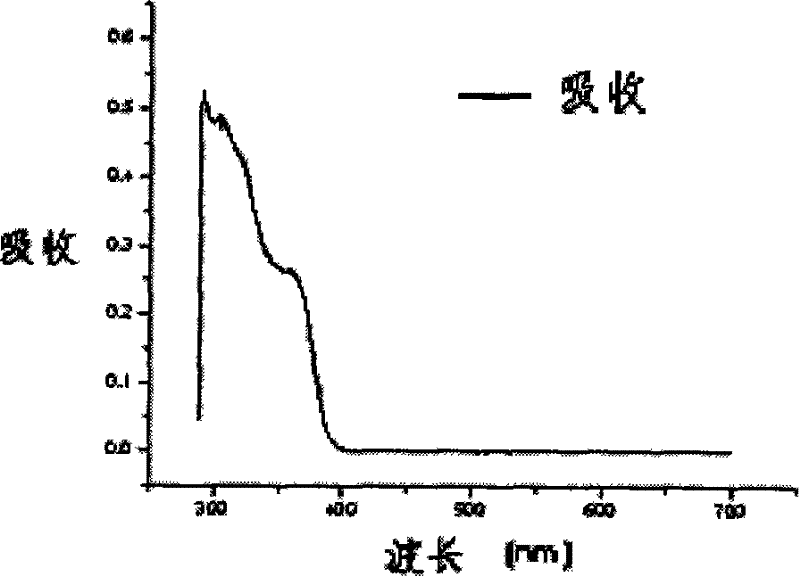
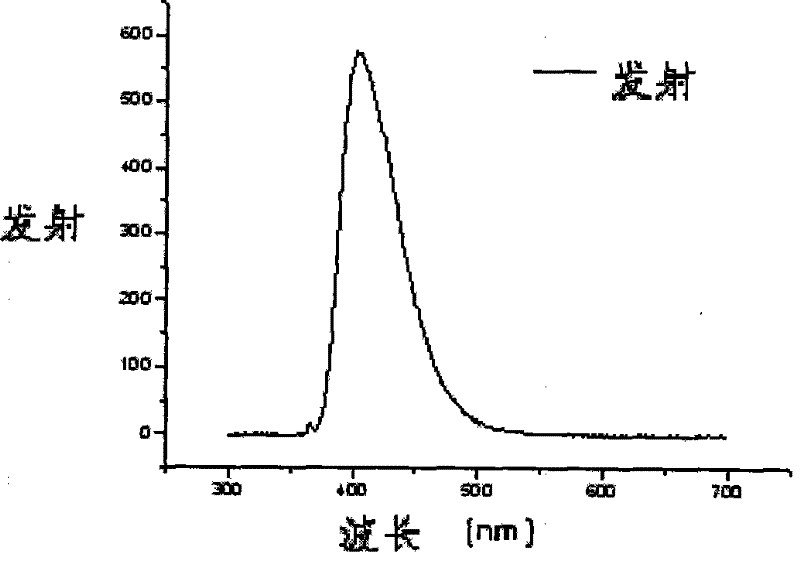
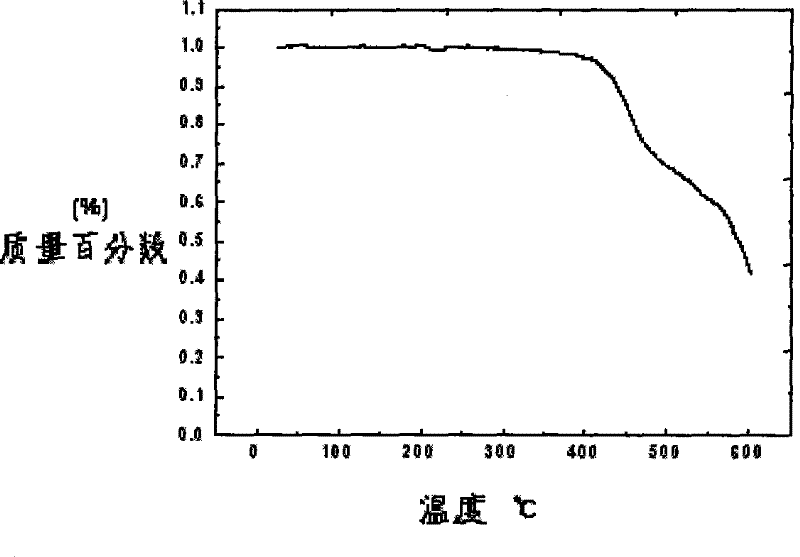
Image
Examples
Embodiment 1
[0055] Embodiment 1, preparation of poly(1,8-carbazole) homopolymer material:
[0056] 9-Ethyl-carbazole
[0057] Mix 5g carbazole, 3g KOH, and 30ml DMSO in a three-necked flask, add 4ml of bromoethane dropwise at 85°C, stir for 3 hours, add 100ml of water after cooling, and leave to stand for a light yellow solid to precipitate, filter, After drying and recrystallization from absolute ethanol, the product was obtained as white needle crystals (yield 90%).
[0058] GC-MS (EI-m / z): 195 (M + ).
[0059] 3,6-di-tert-butyl-9-ethyl-carbazole
[0060] Take 9-ethyl-carbazole (20mmol), 100mlCH 2 NO 3 Mix it with zinc chloride (8.1g, 60mmol) in a three-necked flask, and add tert-butyl chloride (6.5ml, 60mmol) dropwise to the reaction flask under nitrogen gas. After reacting at room temperature for 5 hours, add 200ml of water The reaction was quenched, extracted with dichloromethane, dried, and rotary evaporated to obtain a white product (yield 94%).
[0061] GC-MS (EI-m / z): 307 ...
Embodiment 2、1
[0070] Embodiment 2, 1,8-carbazole and 1,4-benzene unit copolymer material preparation:
[0071] 1,4-bis(4,4,5,5-tetramethyl-[1,3,2]dioxaborolanyl)-benzene
[0072] First, take 1,4-dibromo-benzene (1.0 equiv.) and put it into a 250ml two-necked flask. The flask has been heated, dried and airtight, and then evacuated three times with nitrogen. Oxygen device for processing use. Then, the reaction apparatus was placed in a -78°C low temperature bath generated by dry ice and acetone, and freshly distilled tetrahydrofuran (20 ml) was taken anhydrous and oxygen free. Then n-butyllithium (3.0 equiv.) was slowly added to the two-necked flask, and reacted at a low temperature of -78°C for about 1 hour. Finally, 2-isopropoxy-4,4,5,5- Tetramethyl-1,3,2-dioxaborane (3.0 equiv.) was quickly injected into the reactor, and the reaction was slowly returned to room temperature, and the reaction was carried out overnight. After the reaction was completed, the reaction was quenched with ice w...
Embodiment 3、1
[0076] Example 3, 1,8-carbazole and 9,9-dioctylfluorene unit random copolymer material preparation:
[0077] 2,2'-(9,9-di-n-octanefluorene-2,7-diyl)-bis([1,3,2]dioxaborinane)
[0078] Magnesium turnings (2.1 quiv.) and a little crystalline iodine were added to a 250 ml four-neck flask equipped with a reflux condenser and dropping funnel, which had been heat-dried and purged with nitrogen by evacuation. First, 1 ml of a solution of 2,7-dibromo-9,9-n-octanefluorene (1 equiv.) dissolved in tetrahydrofuran (10 ml) was added dropwise as quickly as possible, and the mixture was heated at the dropping site. After the reaction started, the remaining solution was slowly added dropwise, and the rate of addition was based on the automatic boiling of the reaction mixture. Tetrahydrofuran (ca. 1.101) was then added and the mixture was refluxed for 2h. The resulting clear Grignard solution was then cooled to room temperature and slowly added dropwise to an excess of freshly distilled trii...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com