Break-in conjugated branched polymer material and preparation method and uses thereof
A technology of hyperbranched polymers and polymer materials, applied in chemical instruments and methods, luminescent materials, semiconductor/solid-state device manufacturing, etc., to achieve the effect of high glass transition temperature and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
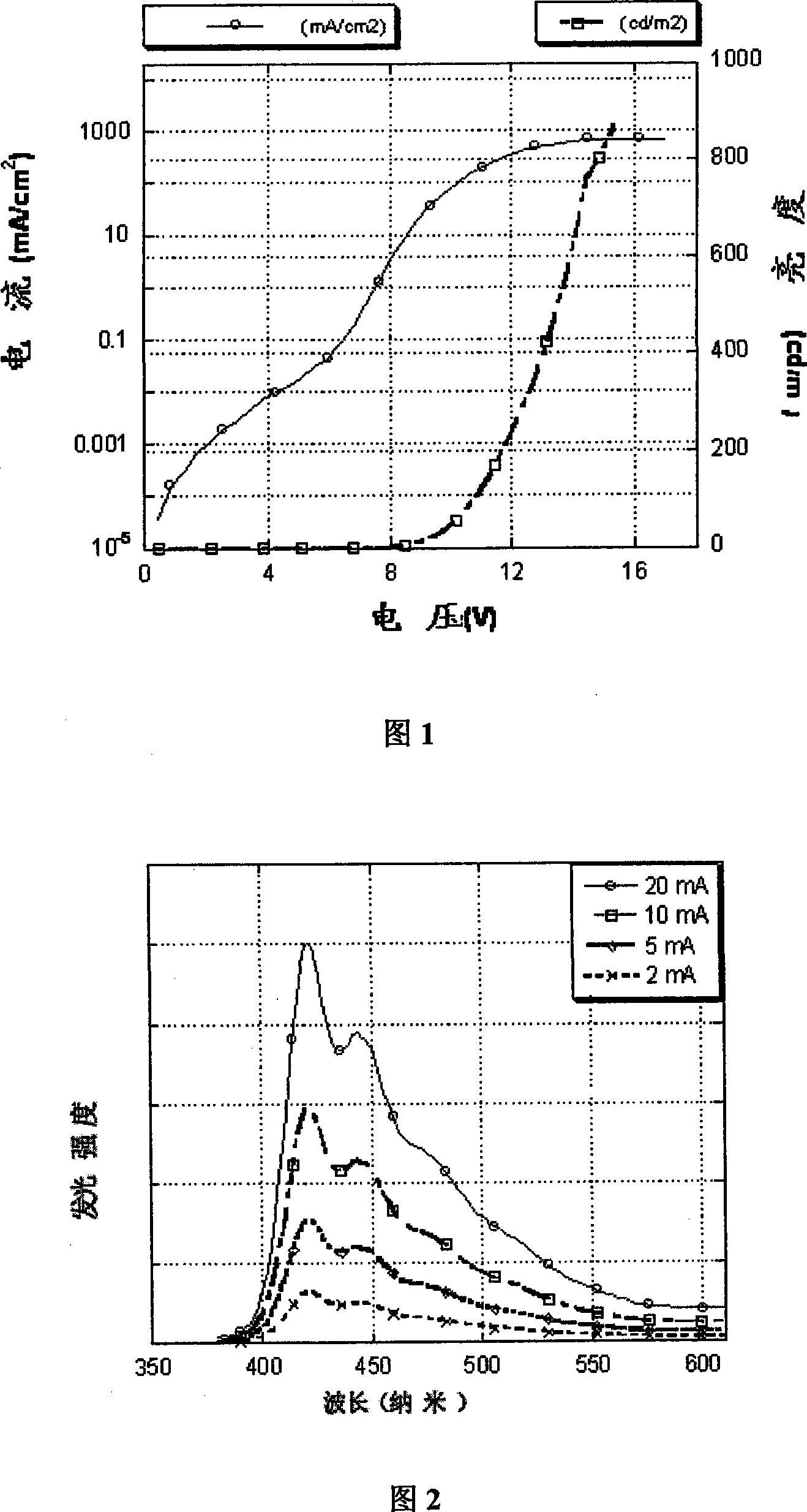
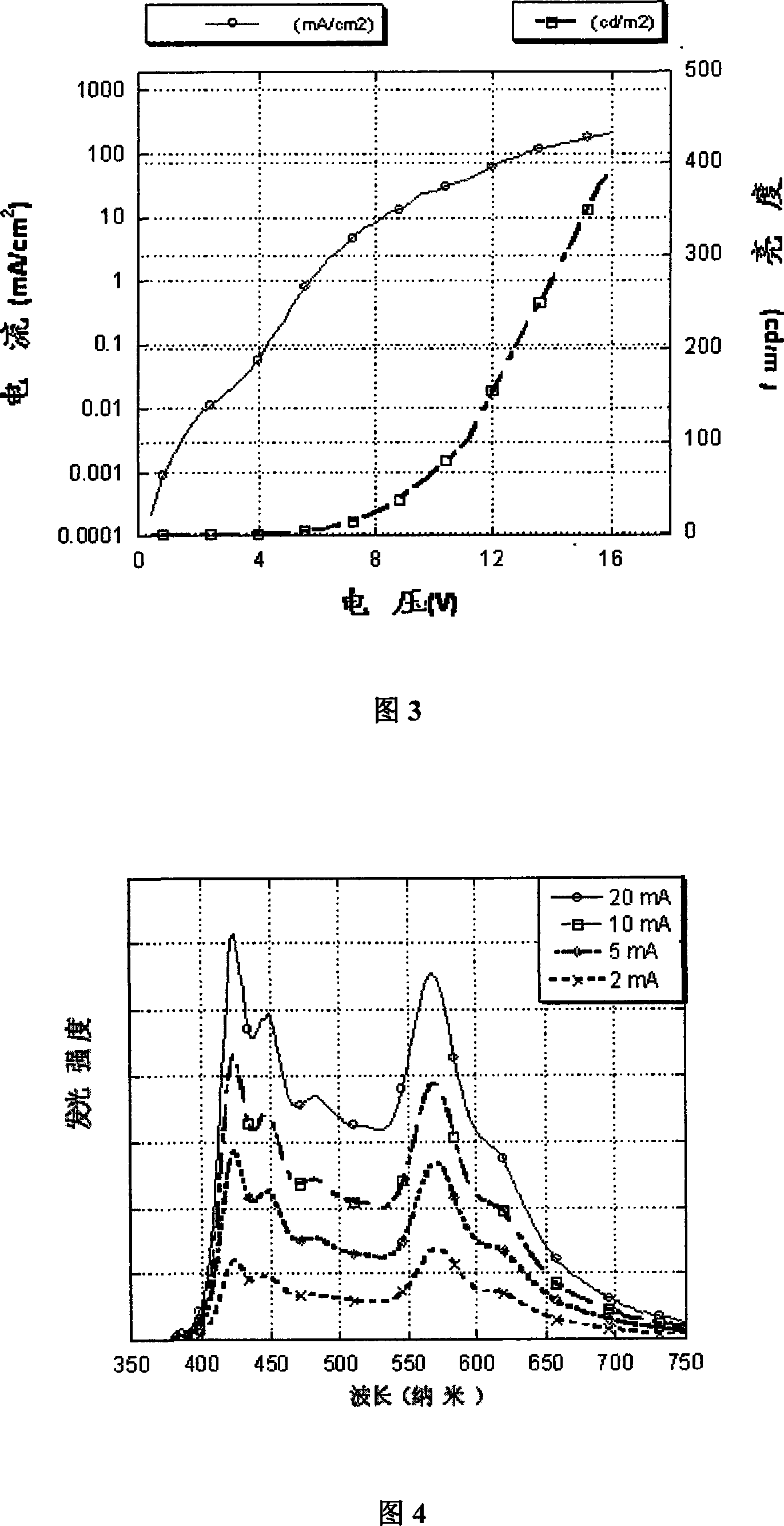
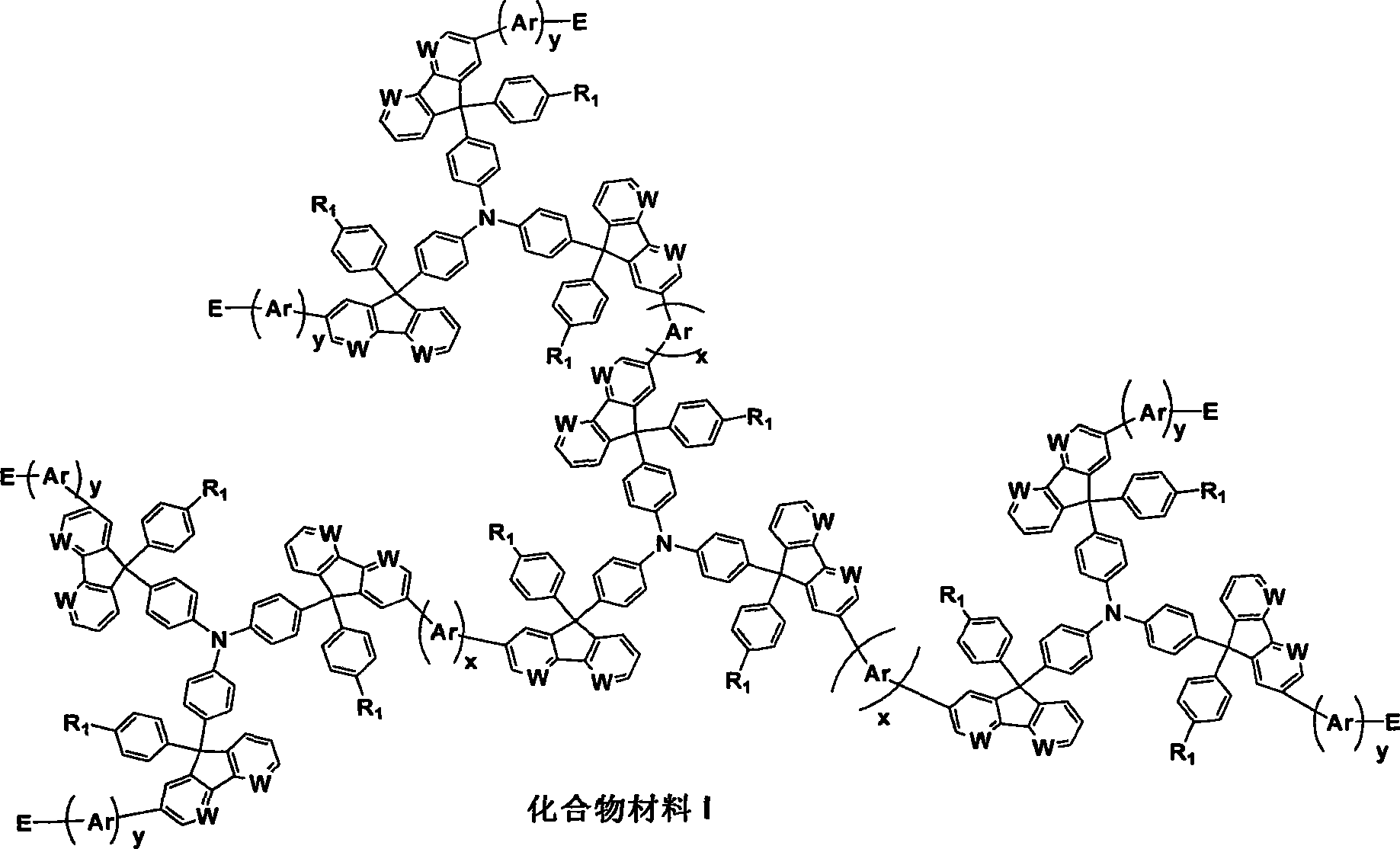
Image
Examples
Embodiment 1
[0036] Example 1, a copolymer material containing 5% of tris(4-(2-bromo-9-phenylfluoren-9-yl)phenyl)amine branched units terminated with pyrene:
[0037] 2-Bromo-9-phenyl-fluoren-9-ol
[0038] 2-bromo-9-phenyl-9H-fluoren-9-ol
[0039] Take bromo-benzene (4.48g, 29mmol) and magnesium Mg (0.58g, 24mmol) to react to generate Grignard reagent, and react with 2-bromofluorenone (3.135g, 12.1mmol) dissolved in tetrahydrofuran (20mL) at 60°C for 24 Hours, a large amount of white precipitate was generated, and finally saturated color NHCl was added 4 Convert Grignard salts to alcohols. After the reaction was completed, extracted with ether, dried and rotary evaporated, and purified on a silica gel column with a mixed solvent of petroleum ether:dichloromethane (3:2) to obtain a slightly pale yellow solid tertiary alcohol (3.70 g, 90%).
[0040] GC-MS (EI-m / z): 334 / 336 (M + ).
[0041] 1 H NMR (400MHz, CDCl 3 , ppm): δ7.657-7.655 (d, J=7.2Hz, 1H), 7.638-7.449 (m, 3H), 7.403-7.35 (...
Embodiment 2
[0061] Example 2, a copolymer material containing 10% of tris(4-(2-bromo-9-phenylfluoren-9-yl)phenyl)amine branched units terminated with pyrene:
[0062] Take tri(4-(2-bromo-9-phenylfluoren-9-yl)phenyl)amine (0.0962g, 0.08mmol), 2,2'-(9,9-di-n-octanefluorene-2, 7-diyl)-bis([1,3,2]dioxaborinane) (0.92mmol) and 2,7-dibromo9,9-dioctylalkanefluorene (0.4387g, 0.8mmol) Mix and dissolve in a mixed solvent of toluene (30mL) and tetrahydrofuran, add catalyst Pd(PPh 3 ) 4 (0.010g, 0.008mmol), avoid light and pass nitrogen, then add K 2 CO 3 (2M, 15mL), reacted under the condition of 75 ℃ for 72 hours, after the reaction, add pyrene boronic acid and catalyst to react for 12 hours, finally, add 1-bromopyrene and catalyst to react for another 12 hours; add hydrazine hydrate (85%) to strongly Stir for 24 hours, extract with toluene, dry and rotary evaporate, and repeatedly reprecipitate by dropping into methanol, and finally use an acetone drawer to obtain a white solid (0.5947g, yiel...
Embodiment 3
[0064] Example 3, a copolymer material containing 15% of tris(4-(2-bromo-9-phenylfluoren-9-yl)phenyl)amine branched units terminated with pyrene:
[0065] Take tris(4-(2-bromo-9-phenylfluoren-9-yl)phenyl)amine (0.0361g, 0.03mmol), 2,2'-(9,9-di-n-octanefluorene-2 ,7-diyl)-bis([1,3,2]dioxaborinane) (0.645mmol) and 2,7-dibromo9,9-dioctylalkanefluorene (0.3291g, 0.6mmol ) are mixed and dissolved in a mixed solvent of toluene (20mL) and tetrahydrofuran, and the catalyst Pd (PPh 3 ) 4 (0.010g, 0.008mmol), avoid light and pass nitrogen, then add K 2 CO 3 (2M, 10mL), reacted under the condition of 75 ℃ for 72 hours, after the reaction, add pyrene boronic acid and catalyst to react for 12 hours, finally, add 1-bromopyrene and catalyst to react for another 12 hours; add hydrazine hydrate (85%) to strongly Stir for 24 hours, extract with toluene, dry and rotary evaporate, repeat reprecipitation by dropping into methanol, and finally use an acetone drawer to obtain a white solid (0.42...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com