Perylene diimide photoelectric functional materials and preparation method thereof
A photoelectric functional material, perylene imide technology, applied in the direction of photovoltaic power generation, circuits, electrical components, etc., can solve the problems of cost reduction of inorganic solar cells, photocorrosion limitations, etc., achieve good solubility, eliminate crystallization and pinholes, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
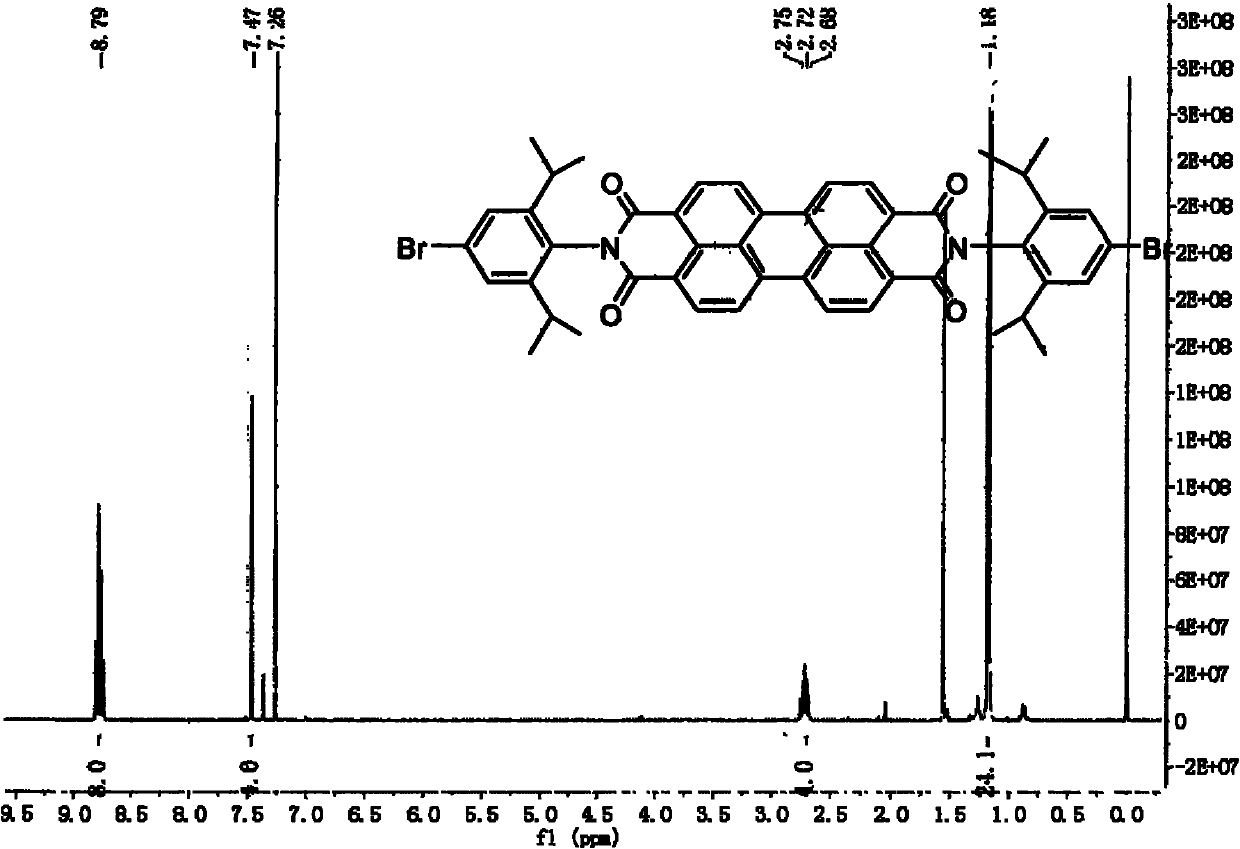
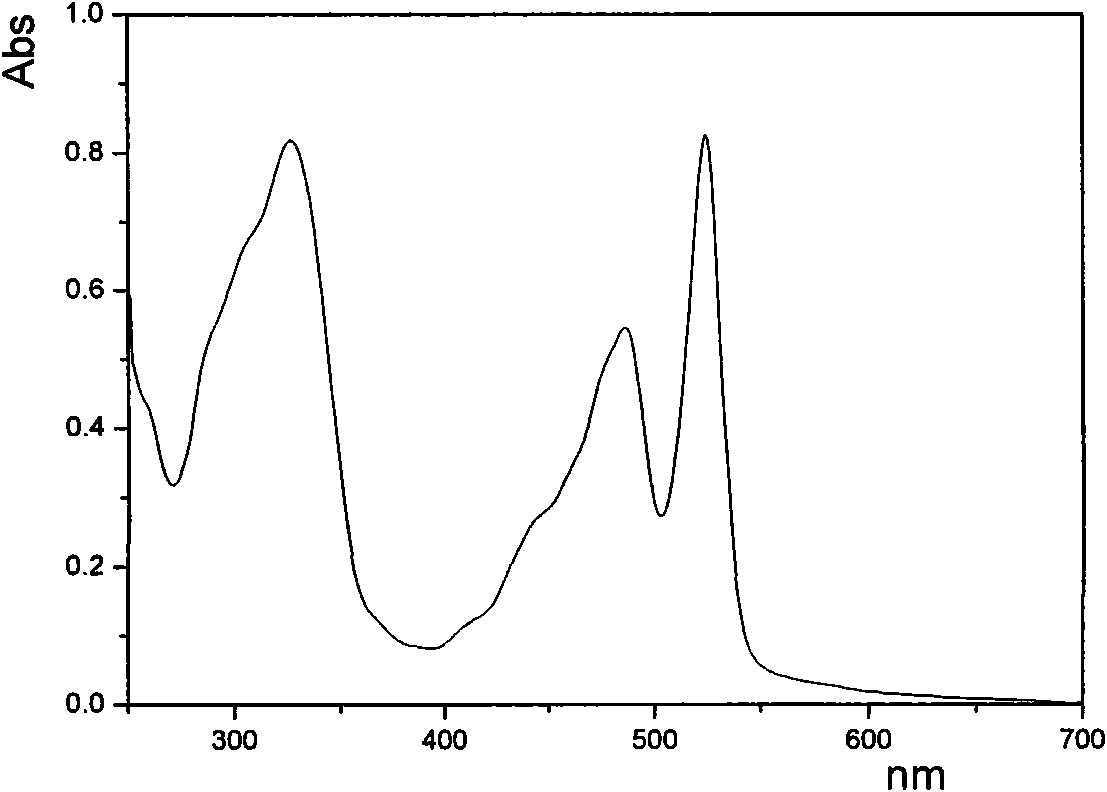
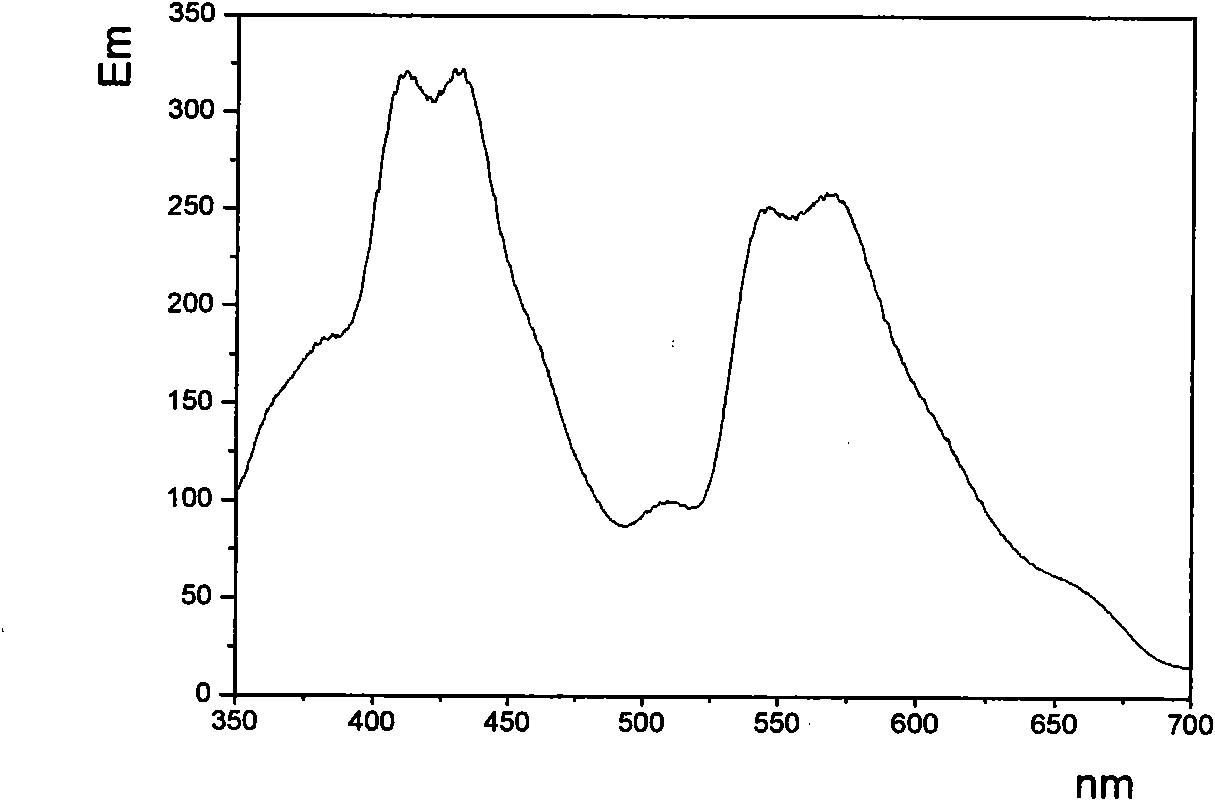
[0041] Example 1, N, N'-diisopropylbromophenylperylenediimide and 9,9'-dioctyl-2,7-dibromofluorene copolymer preparation:
[0042] (1) 2,2'-(9,9-di-n-octanefluorene-2,7-diyl)-bis([1,3,2]dioxaborinane)
[0043] Magnesium turnings (2.1 equiv.) and a little crystalline iodine were added to a 250 ml four-necked flask equipped with a reflux condenser and dropping funnel, which had been heat-dried and purged with nitrogen by evacuation. First, 1 ml of a solution of 2,7-dibromo-9,9-n-octanefluorene (1 equiv.) dissolved in tetrahydrofuran (10 ml) was added dropwise as quickly as possible, and the mixture was heated at the dropping site. After the reaction started, the remaining solution was slowly added dropwise, and the rate of addition was based on the automatic boiling of the reaction mixture. Tetrahydrofuran (ca. 1.10 ml) was then added and the mixture was refluxed for 2 h. The resulting clear Grignard solution was then cooled to room temperature and slowly added dropwise to an ...
Embodiment 2
[0046] Embodiment 2, the preparation of the copolymer of N, N'-dimethyl bromophthalic imide and 9-octyl-3,6-dibromocarbazole:
[0047] (1) Catalyst Ni(COD) 2 preparation of
[0048] Dissolve nickel 1,5-bicyclooctadienide, 1,5-cyclooctadiene and 2,2-bipyridine in dry toluene (3ml) and DMF (3ml) at a molar ratio of 1:1:1 In the mixed solution, replace N 2 , heated at 80°C for 30 minutes with stirring to prepare nickel catalyst
[0049] (2) Preparation of copolymer
[0050] A nickel catalyst (3 equiv.) was added to a 25ml two-necked flask equipped with a reflux condenser, the flask had been heated and dried and nitrogen was vented with vacuum. Add peryleneimide (1equiv.) and 9-octyl-3,6-dibromocarbazole (1equiv.) dissolved in 4ml toluene, heat at 80°C for 12h, add excess bromobenzene and keep heating for 1h, cool down The reactant was poured into a mixed solution of 400ml of toluene and hydrochloric acid (1:1) and stirred for 4 hours, filtered, and the precipitate was purifi...
Embodiment 3
[0051] Embodiment 3, N, the preparation of the copolymer of N'-dimethoxy bromophthalic imide and tertiary thiophene:
[0052] (1) Preparation of triple thiophene tin reagent
[0053] Prepare a dry and clean 25ml two-necked flask, replace the nitrogen, add the THF solution of dibromoterthiophene at -78 degrees, slowly add butyllithium, stir at room temperature for 1 hour, then cool to -78 degrees, slowly add tributyltin chloride The THF solution was stirred at room temperature for 4 hours. After the reaction, the mixture was poured into water, extracted, the organic phase was dried, spin-dried and passed through the column, and purified by recrystallization to obtain a pure triple thiophene tin reagent.
[0054] (2) Preparation of polymer
[0055] Put peryleneimide (1equiv), tertiary thiophene tin reagent (1equiv), tetrakistriphenylphosphorous palladium catalyst (0.02equiv) into a clean dry reaction test tube, add toluene and potassium carbonate saturated solution, under nitro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com