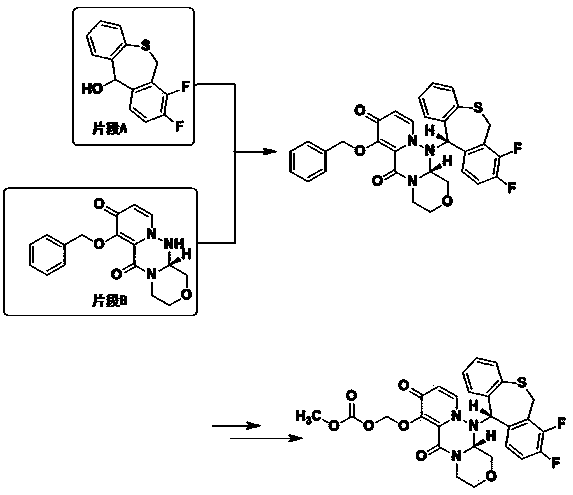
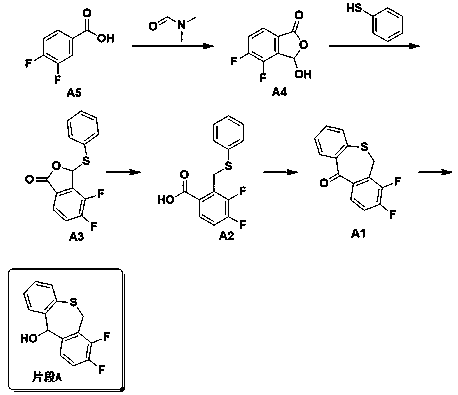
Preparation method of baloxavir marboxil intermediate
A technology for intermediates and solvents, applied in the field of preparation of baloxavir intermediates, can solve problems such as long routes, unsuitable for commercial production of fragments, etc., and achieve the effects of reducing side reactions, reducing harm, and improving reaction yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
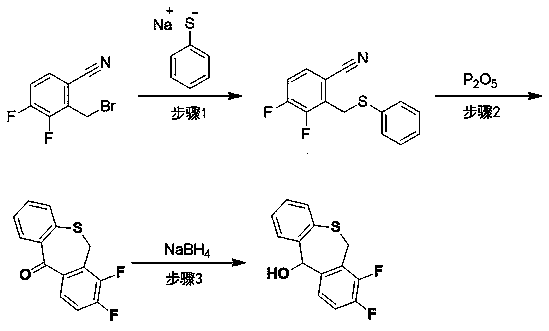
[0023] In a 3L three-necked round-bottomed flask, add 2-bromomethyl-3,4-difluorophenylacetonitrile (100.00g, 430.99mmol), acetone (800ml), stir and clarify, then add sodium thiophenate (113.92 g, 861.98mmol) in water (900ml), triethylamine (91.58g, 905.08mmol). The system was mixed and stirred at room temperature for 5 hours, acetone was distilled off under reduced pressure, extracted with dichloromethane (1L×2), the dichloromethane phases were combined, washed with water, washed with brine, dried over anhydrous sodium sulfate, and rotary evaporated under reduced pressure to obtain a residue 110.00g, yield 97.7%, was directly put into the next reaction without purification.
[0024] In a 5L three-necked flask, add 3,4-difluoro-2-[(phenylthio)methyl]phenylacetonitrile (100.00g, 382.72mmol) produced by the previous step reaction, and phosphorus pentoxide (271.62g, 1.91mol), xylene (2.5L), diatomaceous earth (270.00g), the system was stirred and refluxed for 5 hours, cooled to r...
Embodiment 2
[0029] In a 3L three-neck round bottom flask, add 2-bromomethyl-3,4-difluorophenylacetonitrile (120.00g, 517.20mmol), ethanol (900ml), stir and clarify, then add sodium thiophenate (132.56 g, 1.003mol) in water (900ml), triethylamine (103.57g, 1.024mol). The system was mixed and stirred at room temperature for 5 hours, and the ethanol was distilled off under reduced pressure, then extracted with dichloromethane (1L×2), the dichloromethane phases were combined, washed with water, washed with brine, dried over anhydrous sodium sulfate, and evaporated under reduced pressure to obtain a residue 124.3g, yield 96.9%, was directly put into the next reaction without purification.
[0030] In a 250ml three-necked flask, add 3,4-difluoro-2-[(phenylthio)methyl]phenylacetonitrile (3.00g, 11.47mmol), phosphorus pentoxide (8.12g), xylene (80ml) , diatomaceous earth (8.00g), the system was stirred and refluxed for 4 hours, cooled to room temperature, filtered, washed with water (30ml) of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com