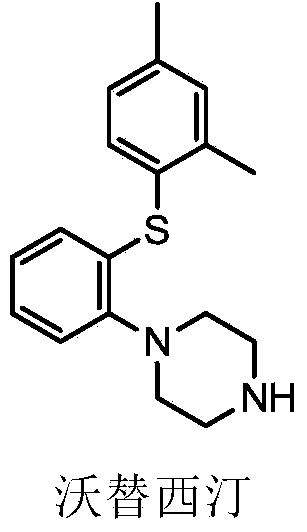
Synthetic method of vortioxetine
A technology of vortioxetine and a synthesis method, applied in the field of medicinal chemistry, can solve problems such as unfavorable commercial production, low product purity, high price, etc., and achieve the effects of avoiding high-cost post-processing, high purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
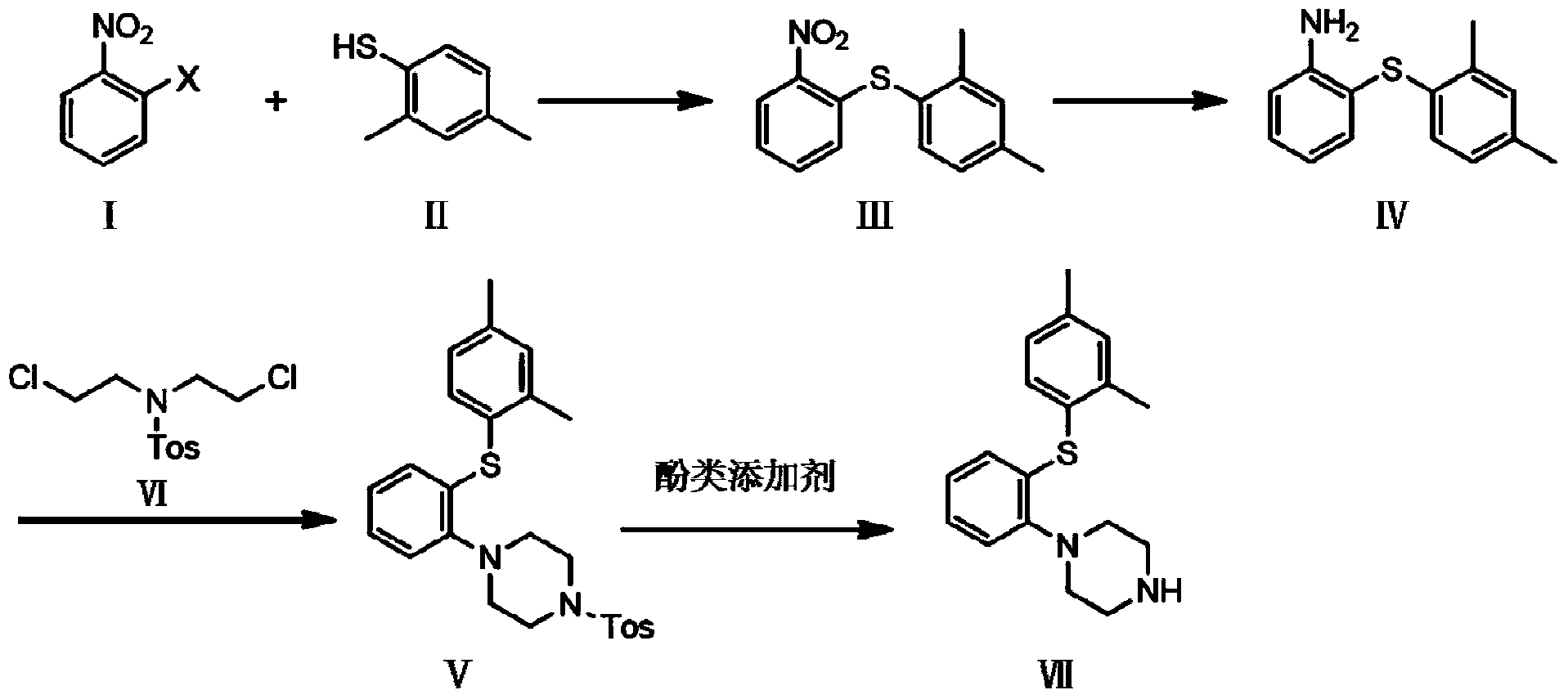
[0038] Embodiment 1 (the first step):
[0039] Add 341.6g of o-chloronitrobenzene (I), 500g of 2,4-dimethylthiophenol (II), 876g of potassium carbonate, and 3L of acetone into the reaction flask, and react under reflux at 65°C for 18h. After stopping the reaction, distill off the solvent, then add 4L of water, stir for 1h, filter with suction to obtain a solid, add 1L of n-heptane: 2N aqueous sodium hydroxide solution = 1L: 1L, stir for 1 hour, filter with suction, and dry at 55°C for 12h ,, 491 g of 2-(2,4-dimethylphenylsulfanyl) nitrobenzene (Ⅲ) was obtained, with a yield of 87.3% and a purity of 95.8%.
Embodiment 2
[0040] Embodiment 2 (the first step)
[0041] Add 438g of o-bromonitrobenzene, 500g of 2,4-dimethylthiophenol, 876g of potassium carbonate, and 3L of acetone into the reaction flask, and react under reflux at 65°C for 18h. After stopping the reaction, distill off the solvent, then add 4L of water, stir for 1h, filter with suction to obtain a solid, add 1L of n-heptane: 2N aqueous sodium hydroxide solution = 1L: 1L, stir for 1 hour, filter with suction, and dry at 55°C for 12h ,, 499 g of 2-(2,4-dimethylphenylsulfanyl) nitrobenzene (Ⅲ) was obtained, with a yield of 88.7% and a purity of 92.7%.
Embodiment 3
[0042] Embodiment 3 (the second step)
[0043] Add 360g of 2-(2,4-dimethylphenylsulfanyl)nitrobenzene (Ⅲ), 233g of iron powder, 297.5g of ammonium chloride, 1.44L of methanol, 1.44L of tetrahydrofuran, and 0.576L of water into the three-necked flask . Replaced with nitrogen, reacted at 60°C for 5.5h. Stop the reaction, let stand for 18h, centrifuge, and then filter with suction, and distill the filtrate to remove the solvent. Add 2L ethyl acetate, dissolve it, add water to wash twice, add 1L each time, then wash with 1L saturated brine, add anhydrous sodium sulfate 500g and dry for 1 hour, evaporate ethyl acetate to obtain the product 2-(2,4 -Dimethylphenylsulfanyl)aniline (IV) 303.0 g, yield 96.8%, purity 96.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com