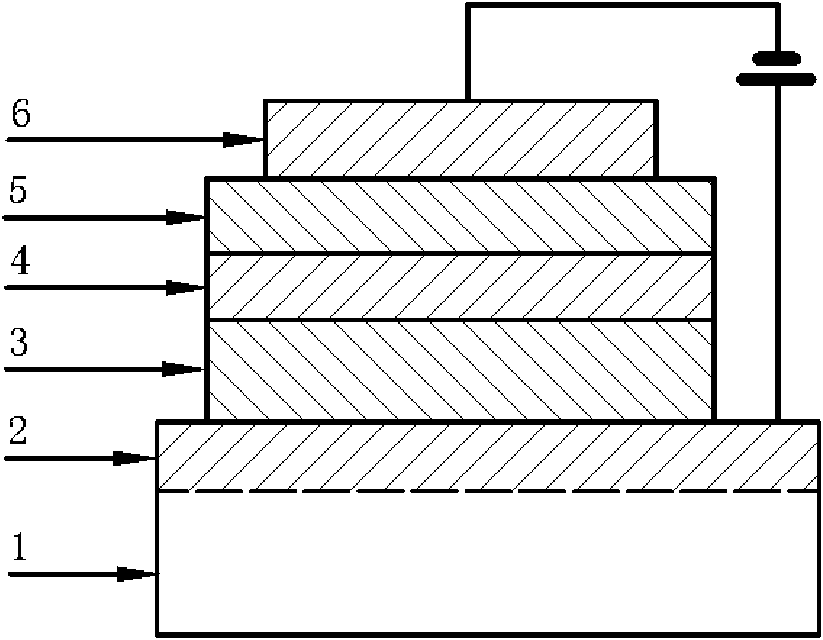
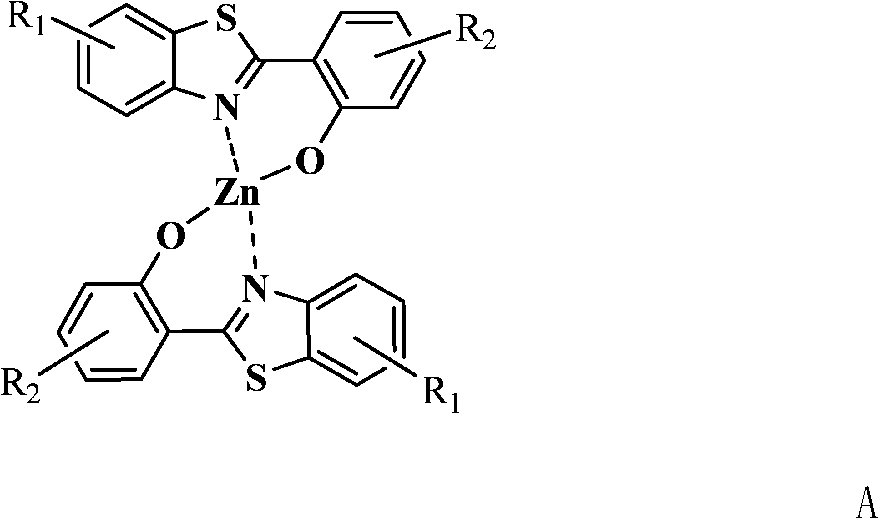
2-(2'-hydroxyphenyl) benzothiazole chelated zinc derivative as well as preparation method and application thereof
The technology of benzothiazole and hydroxyphenyl is applied to 2-(2'-hydroxyphenyl) benzothiazole chelated zinc derivatives and the fields of preparation and application thereof, and can solve the problem of short service life and organic electroluminescence display. The problems of low industrialization process of devices and limited types of electron transport materials have achieved the effect of improving device performance and life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Take 6.1g of 4-methoxyaniline (0.05mol) and 7.6g of o-hydroxybenzoic acid (0.055mol) respectively in a 250ml round bottom flask, and add 100ml of chlorobenzene, and slowly add 3.4g of trichlorobenzene dropwise to the reaction system under stirring. Phosphate (0.025mol). Slowly raise the temperature to 130°C, and reflux at this temperature for 1.5h. After the reaction, an orange-yellow colloidal solid appeared on the wall of the flask, and the solution was a milky white clear liquid. The reaction solution was filtered while hot, and the filtrate was concentrated and then recrystallized with chloroform and methanol (about 1:1). Yield: 88%, Mp 160.2-160.6°C. 1 H NMR (400MHz, CDCl 3 , TMS) δ12.07 (s, 1H), 7.92 (s, 1H), 7.51-7.49 (d, 1H), 7.45-7.40 (m, 3H), 7.02-7.00 (d, 1H), 6.93-6.87 ( m, 3H), 3.80 (m, 3H).
Embodiment 2
[0030] Pack 12.2g N-(4'-methoxyphenyl)-2-hydroxybenzamide (0.05mol), 3.6g imidazole (0.0525mol), and 8.3g tert-butyldimethylsilyl chloride (0.055mol) respectively into a 250ml round bottom flask, and then add 120ml of dichloromethane. Stir magnetically at room temperature for 10 h, and track the reaction with thin-layer chromatography. After the reaction, wash with dilute hydrochloric acid (0.1M) and water three times, and extract with dichloromethane, anhydrous MgSO 4 Dry, filter, and spin out the solvent, and the crude product is directly subjected to thiolation.
[0031] Get Lawson's reagent 12.1g (C 14 h 14 o 2 P 2 S 4 ) (0.03mol) was added to the crude product obtained in the previous step, and then 120ml of toluene was added. After stirring, the temperature was slowly raised to 110° C. under the protection of nitrogen, and the reaction was refluxed at this temperature for 9 hours. Toluene was distilled off under reduced pressure, and a yellow solid was obtained...
Embodiment 3
[0033]
[0034] In the 7.5g of N-(4'-methoxyphenyl)-2-tert-butyldimethylsilyloxythiobenzamide (0.02mol) obtained above, add a small amount of ethanol and fresh 30% NaOH successively solution (0.16mol), then add water to make the NaOH aqueous solution concentration about 10%, and then add the diluted solution dropwise to a 20% K 3 Fe(CN) 6 (0.08mol) aqueous solution, continue to react for 2h after addition. After the reaction, the reaction solution was poured into water, neutralized with dilute hydrochloric acid, and extracted several times with dichloromethane. The organic layers were combined, dried over anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure, and the product was separated and purified by column chromatography. Yield 15%. Mp 150.6-151.1℃. 1 H NMR (400MHz, CDCl 3 , TMS) δ12.492 (s, 1H), 7.86-7.84 (d, 1H), 7.67 (s, 1H), 7.65-7.65 (d, 1H), 7.38-7.34 (m, 1H), 7.31-7.29 ( d, 1H), 7.10-7.08 (d, 1H), 6.96-6.92 (m, 1H). LRMS (ESI, p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com