Specific fluorescence probe for identifying thiophenol and application of specific fluorescence probe
A fluorescent probe, thiophenol technology, applied in the direction of fluorescence/phosphorescence, luminescent materials, chemical instruments and methods, etc., can solve the problems of thiophenol toxicity, hindlimb paralysis, muscle weakness, etc., and achieve the effect of rapid response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 The chemical synthesis of 2-benzothiazolyl-6-(2,4-dinitrophenoxy)naphthalene
[0021] (1) 0.5mmol of 2-benzothiazolyl-6-naphthol and 0.625mmol of potassium carbonate and 0.6mmol of 2,4-dinitrobromobenzene were dissolved in 10mL of N,N dimethylformamide, 80 ℃ for 12 hours;
[0022] (2) The reaction solution is distilled under reduced pressure to obtain a light yellow solid residue;
[0023] (3) The solid was purified by silica gel chromatography and eluted with ethyl acetate-n-hexane (1:3 v / v) to obtain 188 mg of light yellow solid powder. 1 H NMR (400MHz, DMSO) δ8.96(d, J=2.2Hz, 1H), 8.81(s, 1H), 8.48(dd, J=9.2, 2.3Hz, 1H), 8.37(d, J=8.9Hz ,1H), 8.30(d,J=8.5Hz,1H), 8.21(d,J=8.0Hz,1H), 8.11(d,J=7.5Hz,2H), 7.89(s,1H), 7.59(t , J=7.6Hz, 2H), 7.51(t, J=7.4Hz, 1H), 7.37(d, J=9.2Hz, 1H).
Embodiment 2
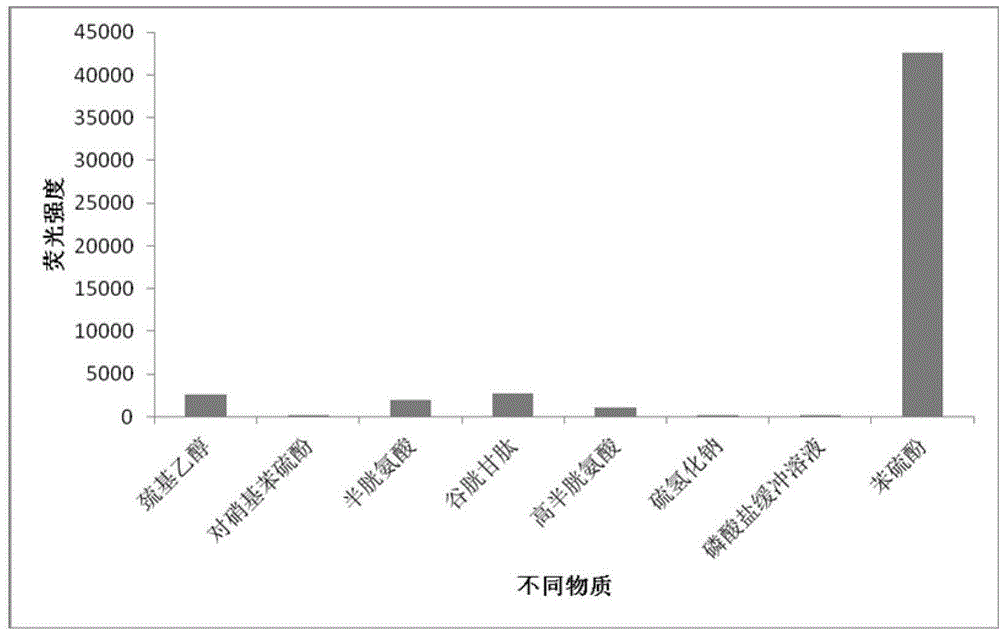
[0024] Example 2 The selectivity of 2-benzothiazolyl-6-(2,4-dinitrophenoxy)naphthalene to different substances
[0025] (1) Prepare 99 μL metabolic reaction system in advance, including PBS buffer (10 mM) at pH 7.4: DMF (volume ratio 1:1), mercaptoethanol (100 μM), p-nitrothiophenol (100 μM), cysteine (100μM), homocysteine (100μM), glutathione (100μM), sodium hydrosulfide (100μM), thiophenol (100μM);
[0026] (2) Add 1 μL of 2-benzothiazolyl-6-(2,4-dinitrophenoxy)naphthalene at a final concentration of 10 μM to the reaction system to initiate the reaction;
[0027] (3) After 20min, carry out fluorescence detection, calculate the fluorescence intensity in each system (see figure 1 ).
Embodiment 3
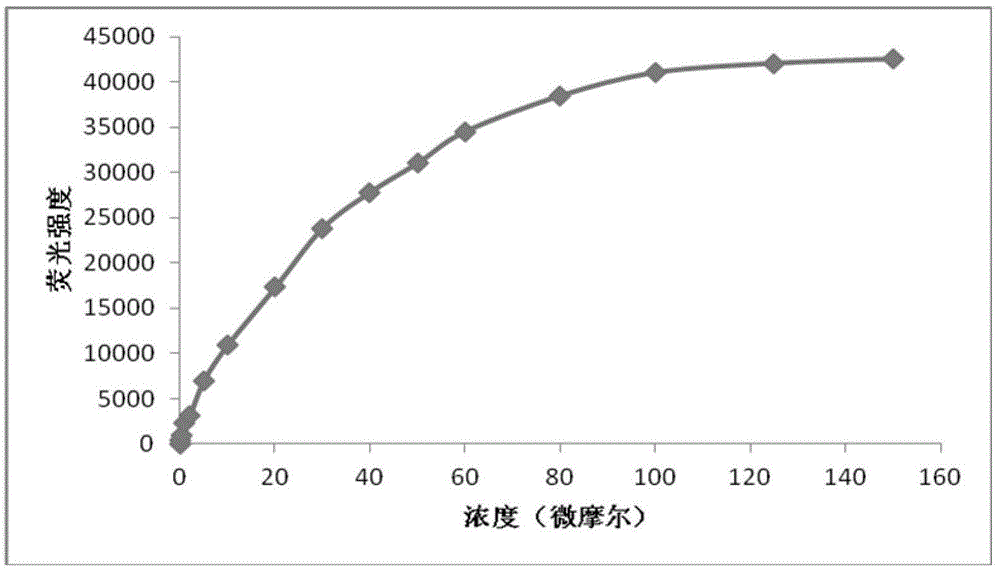
[0028] Example 3 Linear relationship between the concentration of 2-benzothiazolyl-6-(2,4-dinitrophenoxy)naphthalene and thiophenol
[0029] (1) Prepare 99 μL of metabolic reaction system in advance, including PBS buffer (10 mM) at pH 7.4: DMSO (volume ratio 1:9), thiophenol (0-200 μM), and react at 25°C for 30 minutes;
[0030] (2) Add 1 μL of 2-benzothiazolyl-6-(2,4-dinitrophenoxy)naphthalene at a final concentration of 10 μM to the reaction system to initiate the reaction;
[0031] (3) After 20 min, perform fluorescence detection, and calculate the fluorescence intensity in each system. (See figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com