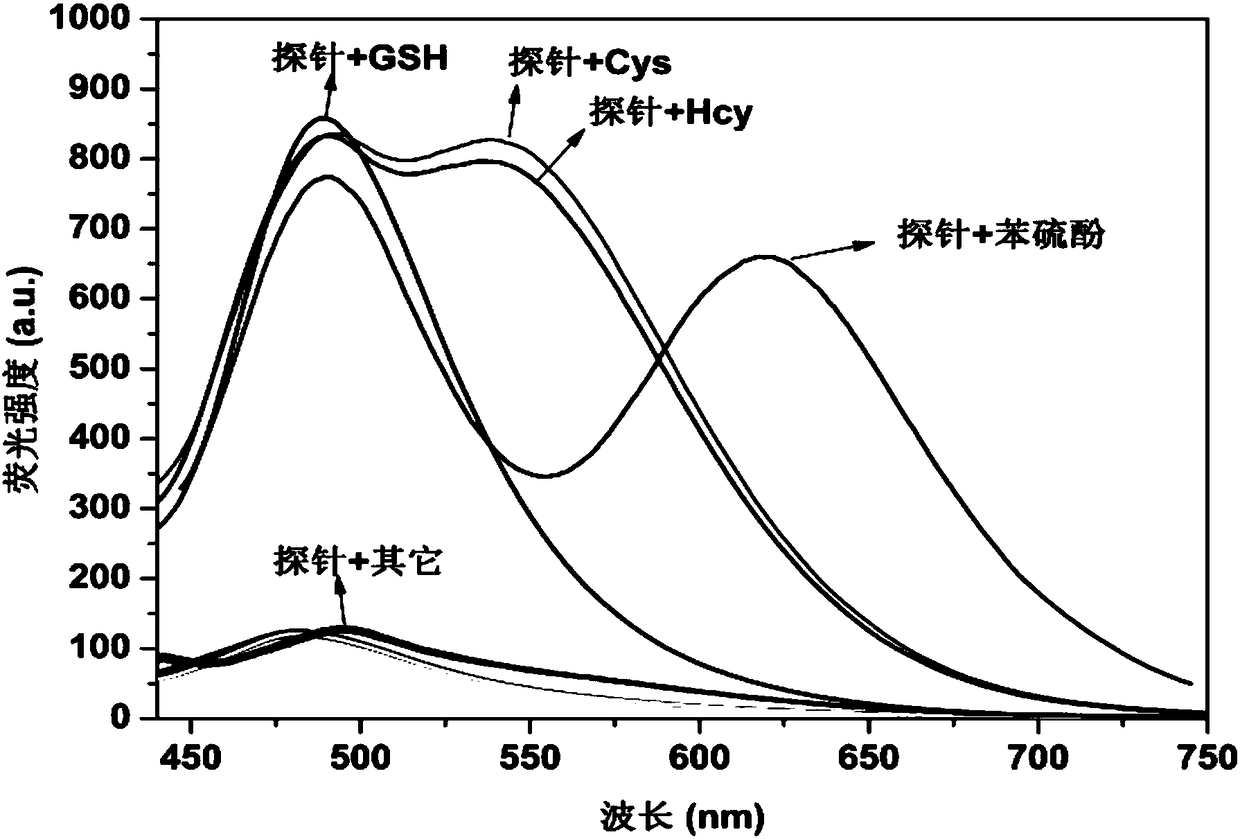
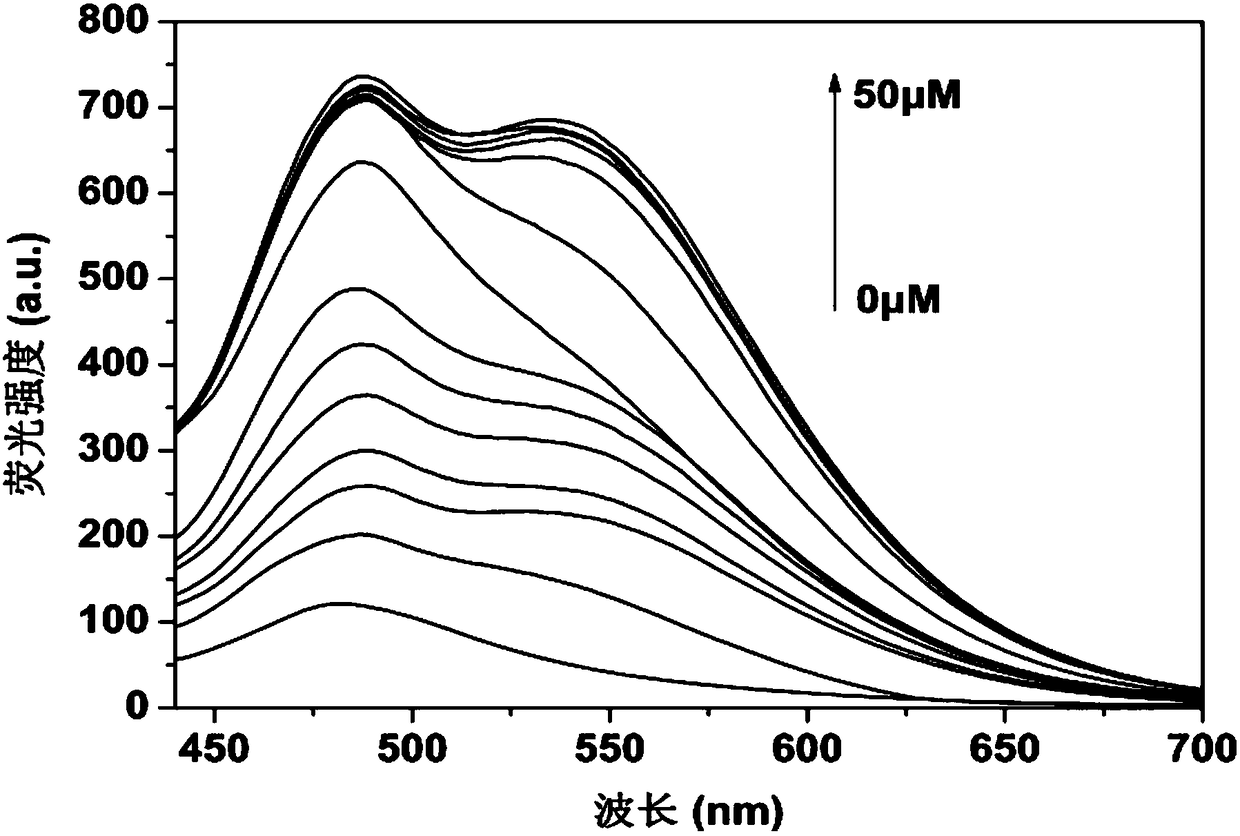
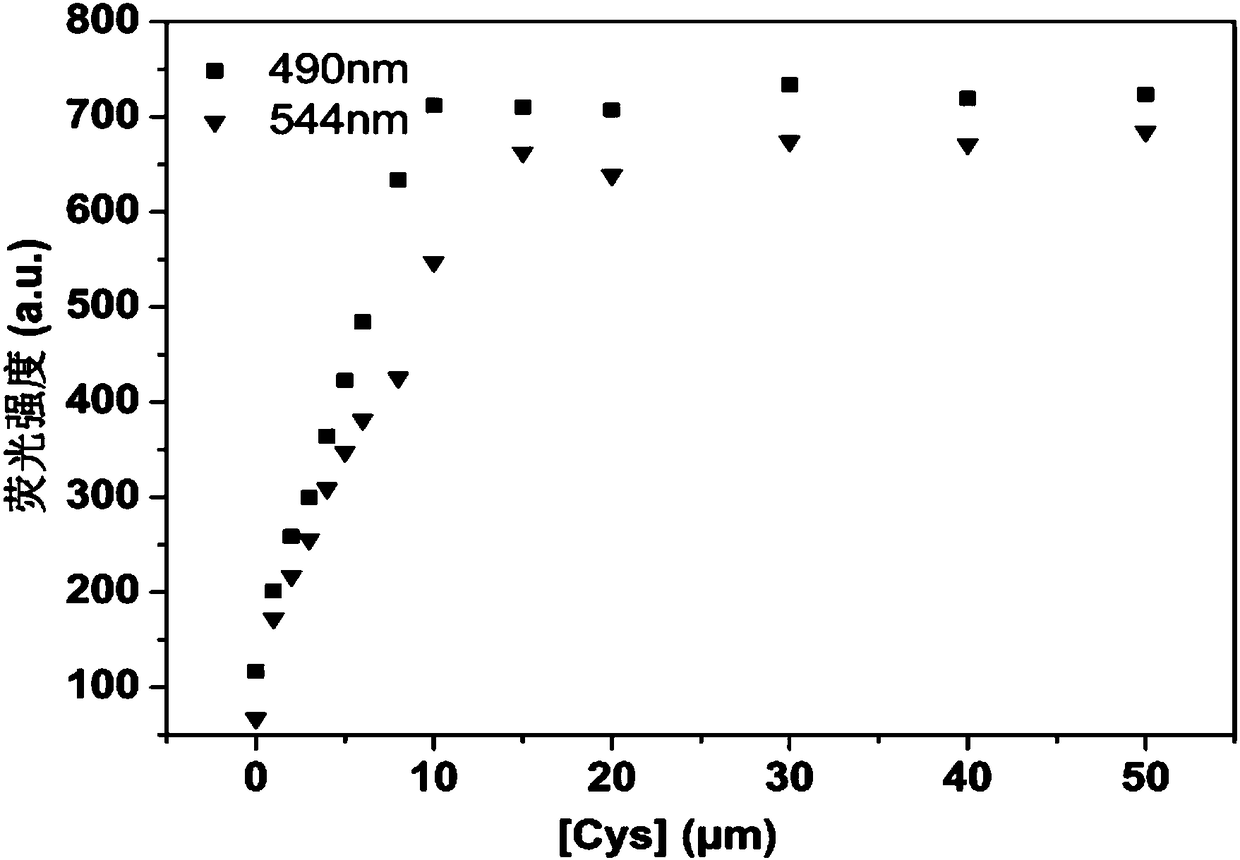
Fluorescent probe for specifically distinguishing different thiol
A fluorescent probe and specific technology, which is applied in the field of fluorescent probes, can solve the problems of poor selectivity, few reports on fluorescent probes, inability to selectively distinguish thiophenols, etc., and achieve simple synthesis routes and simple operations , low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the synthesis of intermediate product 2
[0038] Compound 1 (500mg, 2.6mmol) was dissolved in 15mL of toluene, then cyanoacetic acid (440mg, 5.2mmol) was added, the reaction was refluxed for 3h, and the reaction was stopped. The reaction liquid was cooled to room temperature, filtered with suction, and the filter cake was washed with dichloromethane to obtain 490 mg of beige powder as Compound 2 with a yield of 73.1%. HRMS(ESI)m / z: theoretical value C 13 h 8 NO 5 [M-H] + , 258.0408; experimental value, 258.0420. 1 H NMR (400MHz, DMSO) δ7.59(d, J=8.7Hz, 1H), 6.81(dd, J=8.7, 2.1Hz, 1H), 6.76(d, J=2.1Hz, 1H), 6.31(s ,1H), 5.44(s,2H), 4.23(s,2H), 3.86(s,1H). 13 C NMR (101 MHz, DMSO) δ 166.2, 164.5, 161.9, 160.6, 155.5, 150.1, 126.5, 113.6, 109.4, 108.6, 102.9, 63.2, 25.0.
Embodiment 2
[0039] Embodiment 2: the synthesis of compound CQ
[0040] Compound 3 (102 mg, 0.25 mmol) and piperidine (20 μL) were dissolved in 7.5 mL of dichloromethane, compound 2 (97 mg, 0.37 mmol) was dissolved in 7.5 mL of ethanol, and the two solutions were mixed. The reaction was carried out at 45° C. for 10 h under the protection of argon. After the reaction was finished, the solvent was spin-dried, and the residue was separated and purified by column chromatography. First, dichloromethane and ethyl acetate were used as eluent (V:V=15:1), and then dichloromethane and methanol were used as eluent (V : V=15:1) to perform column chromatography to obtain 50 mg of red powder as compound CQ, with a yield of 30.1%. HRMS(ESI)m / z: theoretical value C 32 h 26 N 5 o 10 [M-H] + , 640.1680; experimental value, 640.1666. 1 H NMR(400MHz,DMSO)δ10.61(s,1H),8.83(d,J=2.4Hz,1H),8.42(dd,J=9.3,2.4Hz,1H),8.14(s,1H),7.62 (s,1H),7.52(d,J=9.3Hz,1H),7.14(d,J=9.3Hz,1H),6.82–6.67(m,2H),6.61(s,1H),6.12(...
Embodiment 3
[0041] Embodiment 3: the synthesis of probe
[0042] Compound CQ (64 mg, 0.1 mmol) was dissolved in 10 mL of acetone, then NBD-Cl (40 mg, 0.2 mmol) and potassium carbonate (60 mg, 0.4 mmol) were added, and stirred at room temperature for 3 h. The reaction was stopped, the solvent was spin-dried, and the residue was separated and purified by column chromatography, using dichloromethane and ethyl acetate (V:V=20:1) as eluents to obtain 21 mg of purple-red powder as probe NCQ. The yield was 26.4%. HRMS(ESI)m / z: theoretical value C 38 h 27 N 8 o 13 [M-H] + , 803.1698; experimental value, 803.4059. 1 H NMR (400MHz, DMSO) δ8.86(d, J=2.8Hz, 1H), 8.67(d, J=8.3Hz, 1H), 8.43(dd, J=9.3, 2.8Hz, 1H), 8.17(s ,1H),7.93(d,J=8.8Hz,1H),7.65–7.57(m,2H),7.40(dd,J=8.8,2.4Hz,1H),7.16(d,J=9.3Hz,1H) ,7.03(d,J=8.3Hz,1H),6.63(s,1H),6.42(s,1H),5.54(s,2H),3.59(d,J=4.9Hz,2H),3.43(dd, J=14.1, 7.0Hz, 2H), 1.31–1.22(m, 4H), 1.18(t, J=7.0Hz, 3H), 1.06(t, J=7.0Hz, 3H). 13 C NMR (101MHz, DMSO) δ163.3,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com