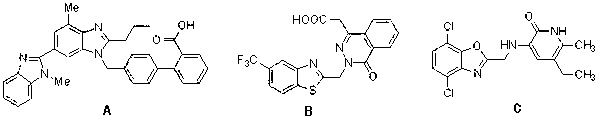
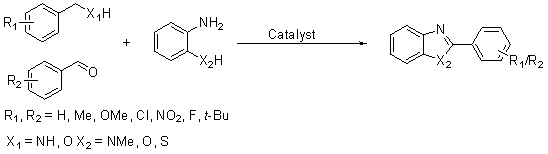
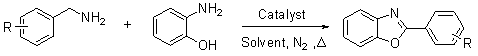Method for preparing 2-substituted benzoxazole compound
A compound and benzoxazole technology, applied in the synthesis field of benzoxazole compounds, can solve problems such as unfavorable environment, troublesome product purification, unfavorable post-processing, etc., and achieve the effects of high atom economy, convenient post-processing, and easy recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A method for preparing 2-substituted benzoxazole compounds, comprising the following steps:
[0034] (1) Mixing of raw materials
[0035] Using benzylamine and o-aminophenol as raw materials, metal palladium (palladium / carbon 53mg) as catalyst, and N,N-dimethylacetamide as solvent: 107mg (1mmol) of benzylamine and 109mg (1mmol) of o-aminophenol , palladium / carbon 53mg (0.05mmol), N,N-dimethylacetamide 2mL were successively added into a three-necked flask, stirred under a nitrogen atmosphere, and the raw materials were mixed with a catalyst and a solvent.
[0036] (2) Reaction
[0037] The mixture in step (1) is subjected to a chemical reaction, the reaction temperature is 120°C (oil bath temperature), and the reaction time is 28h; the chemical reaction formula is:
[0038] .
[0039] (3) Separation and extraction
[0040] After the reaction, the catalyst was filtered off, water and ethyl acetate were added to the filtrate, and the layers were extracted; the aqueou...
Embodiment 2
[0046] A method for preparing 2-substituted benzoxazole compounds, comprising the following steps:
[0047] (1) Mixing of raw materials: benzylamine 107mg (1mmol), o-aminophenol 109mg (1mmol), RuCl 3 Add 10mg (0.03mmol) and 2mL of N,N-dimethylformamide into a three-necked flask in sequence, stir under nitrogen atmosphere, and mix the raw materials with the catalyst and solvent.
[0048] (2) Reaction: The mixture in step (1) is subjected to a chemical reaction, the reaction temperature is 120°C (oil bath temperature), and the reaction time is 38h.
[0049] (3) Separation and extraction: After the reaction, cool down, add water and ethyl acetate to the reaction liquid, extract and separate layers, wash the water phase with ethyl acetate, and combine the organic phases.
[0050] (4) Drying and concentration: the organic phase obtained in step (3) was dried and concentrated with anhydrous sodium sulfate, and the target product——2-substituted benzoxazole compound (2-phenylbenzo ...
Embodiment 3~11
[0054] Under the same experimental conditions and operations as in Example 1, different metal palladium, platinum or ruthenium catalyzed the reaction of benzylamine compounds with o-aminophenol, see Examples 3-11 in Table 1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com