Near-infrared fluorescent probe for detecting thiophenol and synthesis method and application thereof
A synthesis method and a fluorescent molecular probe technology are applied to a near-infrared trun-on type fluorescent probe for highly selective detection of thiophenol, the application field of detecting thiophenol, and can solve unfavorable complex environmental samples and biological samples Detection, weak biological penetration ability, biological damage and other problems, to achieve the effect of high sensitivity, high selectivity and fast response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Synthesis of Fluorescent Molecular Probes
[0035] Compound 2 (0.385g, 1mmol), 2,4-dinitrofluorobenzene (0.186g, 1mmol) was added to K 2 CO 3 (0.685g, 5mmol), the solvent acetonitrile (20mL) was reacted at 80°C for 8h. After the reaction was completed, the solvent was distilled off under reduced pressure, and the product was separated by column chromatography (dichloromethane as the eluent) to obtain 0.473 g of the product as a red-black solid (yield: 86%). The product structural formula is as follows:
[0036]
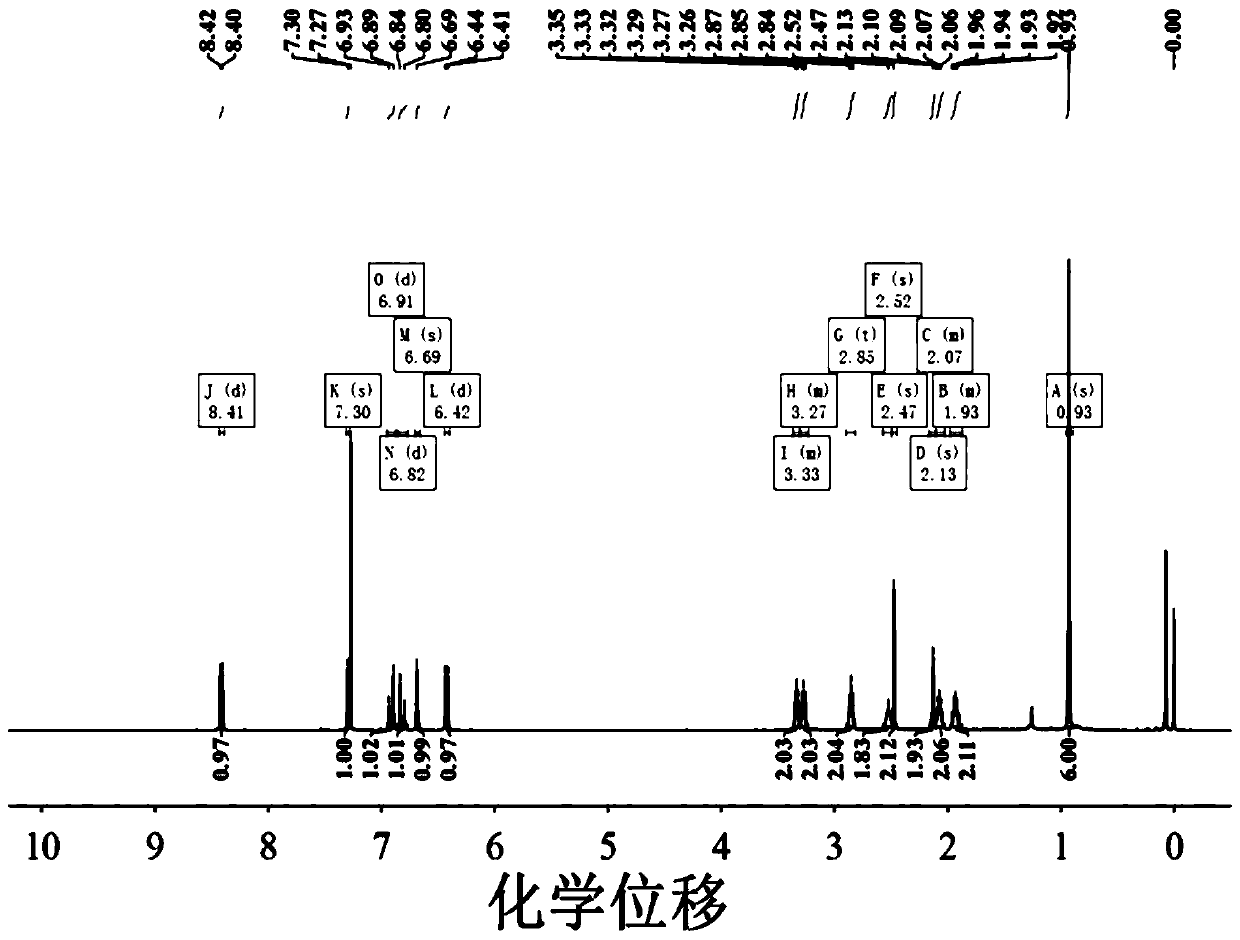
[0037] 1 H NMR (400Hz, CDCl 3 ): δ8.70(d, J=8.1Hz, 1H), 8.05(d, J=8.7Hz, 1H), 7.98(d, J=7.8Hz, 1H), 7.68(ddd, J=32.6, 20.0, 11.5Hz, 5H), 7.50(d, J=8.5Hz, 2H), 7.23(d, J=16.2Hz, 1H), 6.64(d, J=8.4Hz, 2H), 2.86(s, 6H).MS [ESI]:m / z,calcd for[M+H] + 552.22; found 522.14.
Embodiment 2
[0038] Example 2: Fluorescent detection of probes to thiophenol
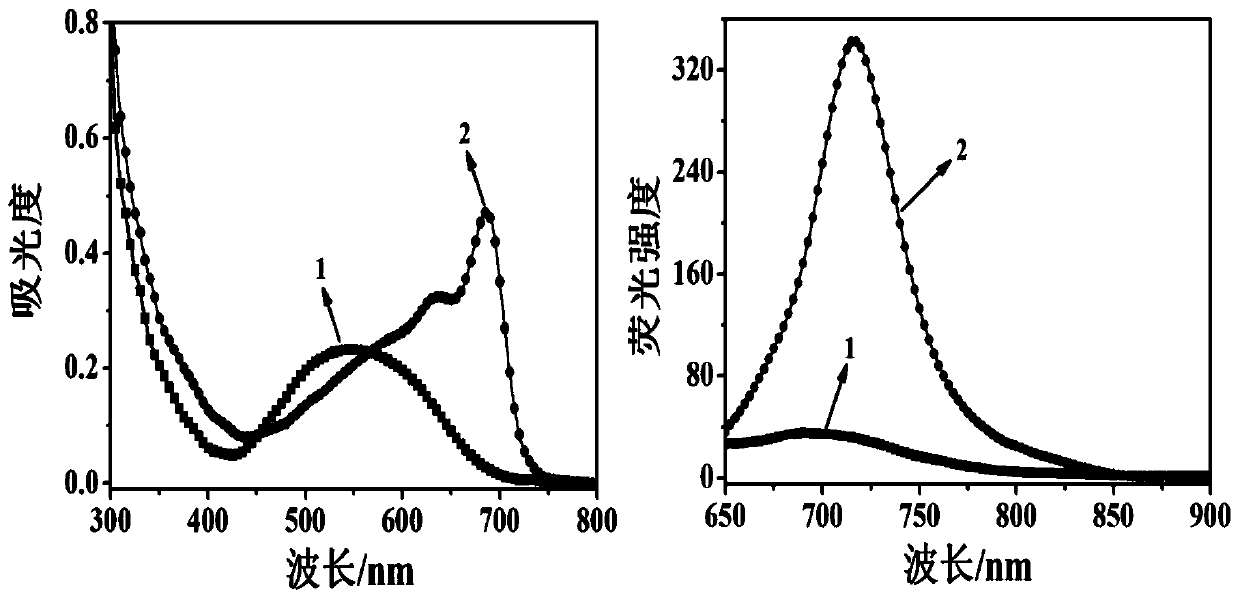
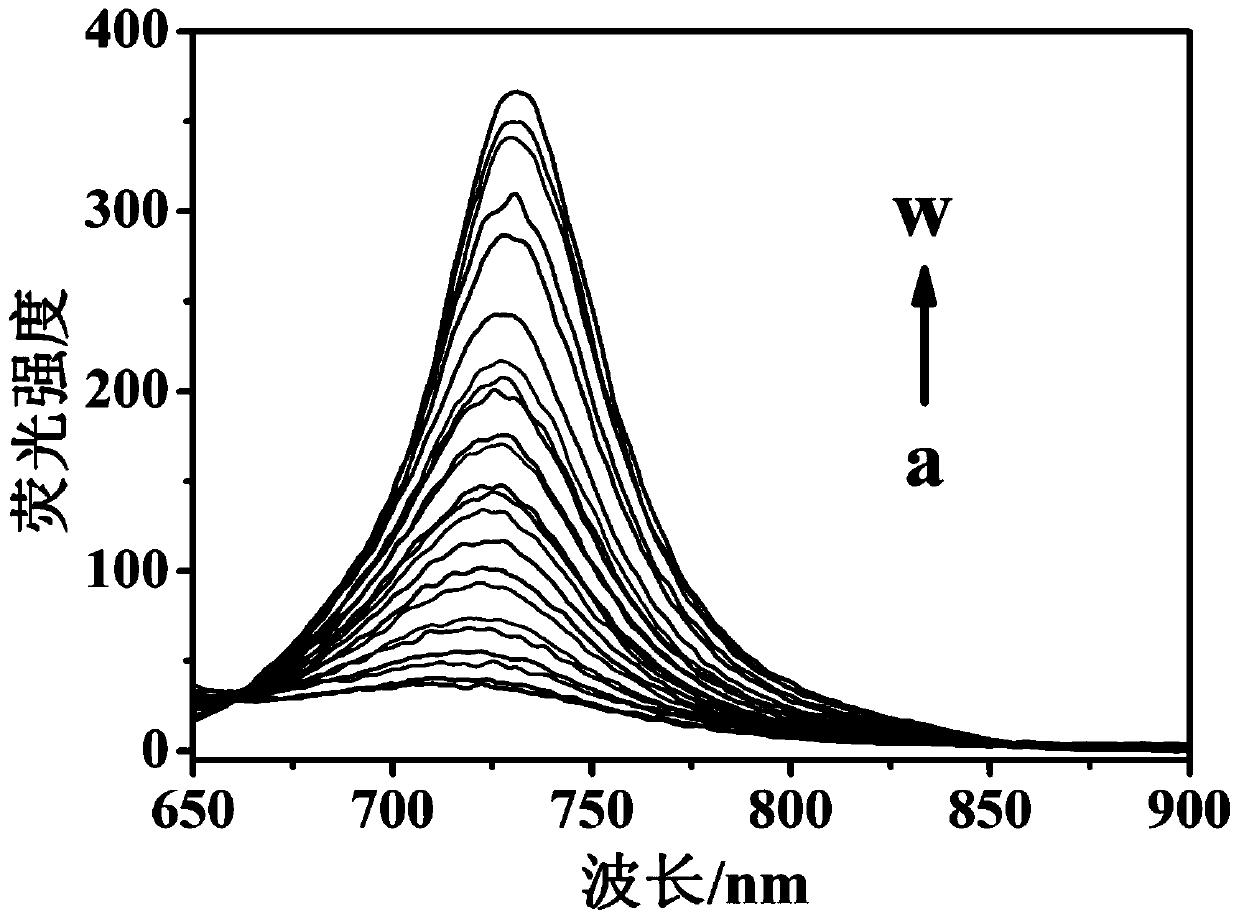
[0039] The molecular probe prepared above was dissolved in a phosphate buffered solution of water and dimethyl sulfoxide (H 2 O / DMSO=1 / 1, v / v, 10mM, pH 7.4), prepared to 10μmol L -1 probe solution. Add 2 mL of prepared 10 μmol L to a 3 mL cuvette -1Probe solution of the present invention, then add different concentrations of thiophenol and mix evenly, test its fluorescence spectrum, the results are as follows image 3 shown. The fluorescence emission intensity of the solution at 730nm is plotted against the concentration of thiophenol, and the concentration of thiophenol is 1–60 μmol L -1 In the range, there is a good linear relationship between the two ( Figure 4 ), the quantitative detection of thiophenol in this concentration range can be realized, and the solution changes from red to blue, which is also suitable for naked eye detection. And this probe is not affected by some other common substances, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com