Fluorescent probe containing N, N-diethyl p-thylaminophenol and application thereof in thiophenol detection
A fluorescent probe and rhodol technology, applied in the direction of fluorescence/phosphorescence, luminescent materials, chemical instruments and methods, etc., can solve the problems of inability to quickly detect thiophenol, inconvenient popularization and application, cumbersome operation, etc., and achieve accurate High accuracy, high sensitivity, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
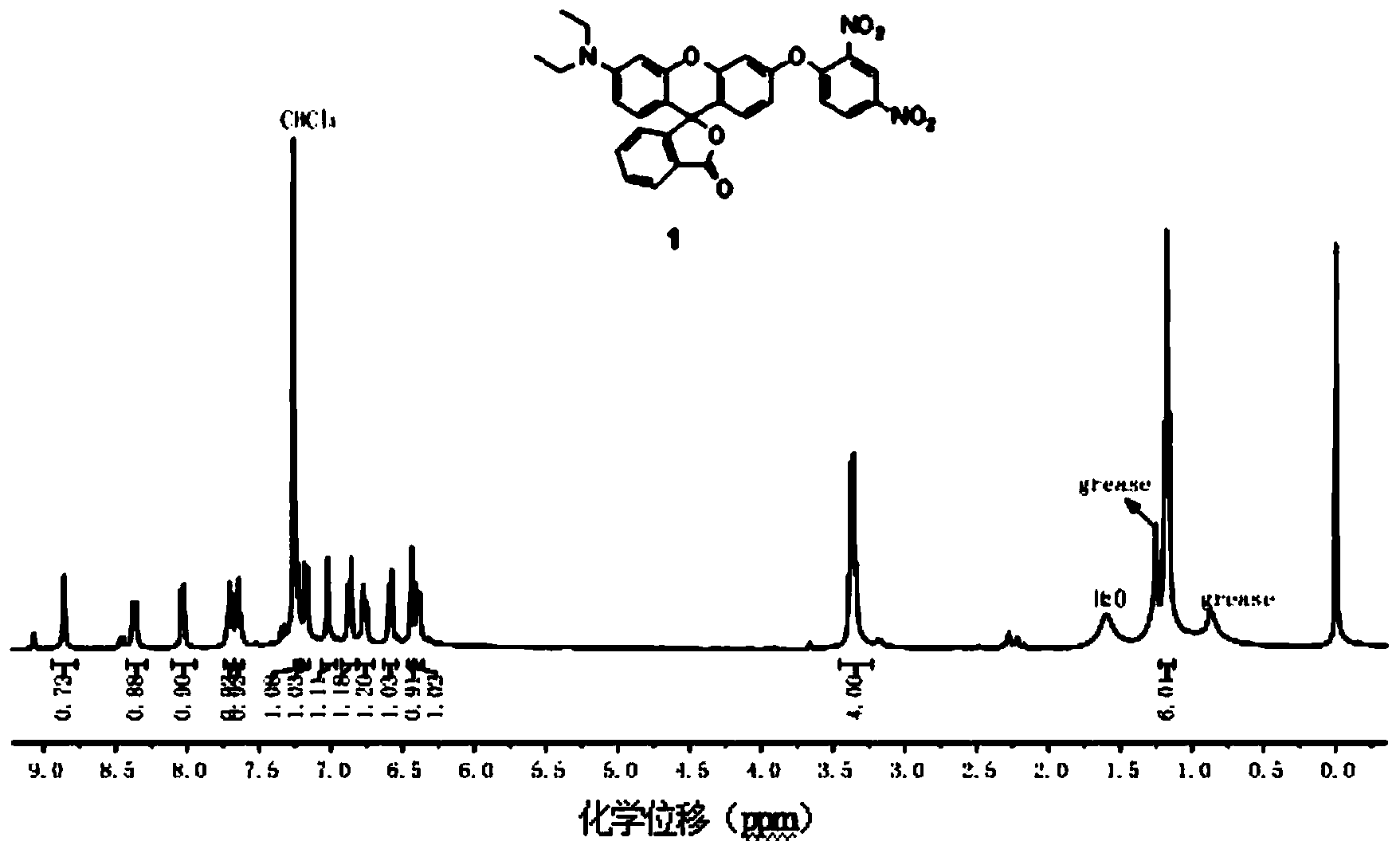
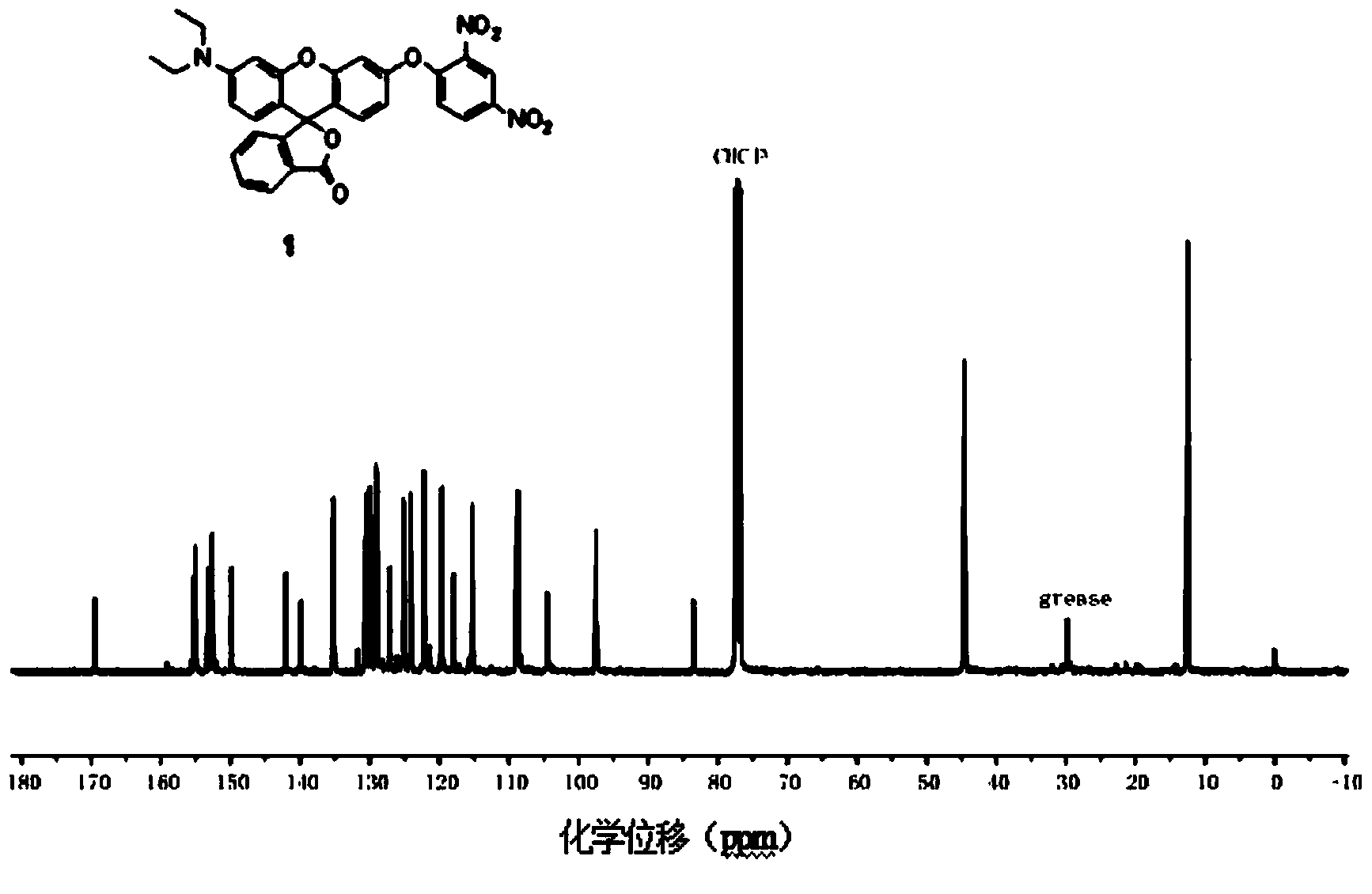
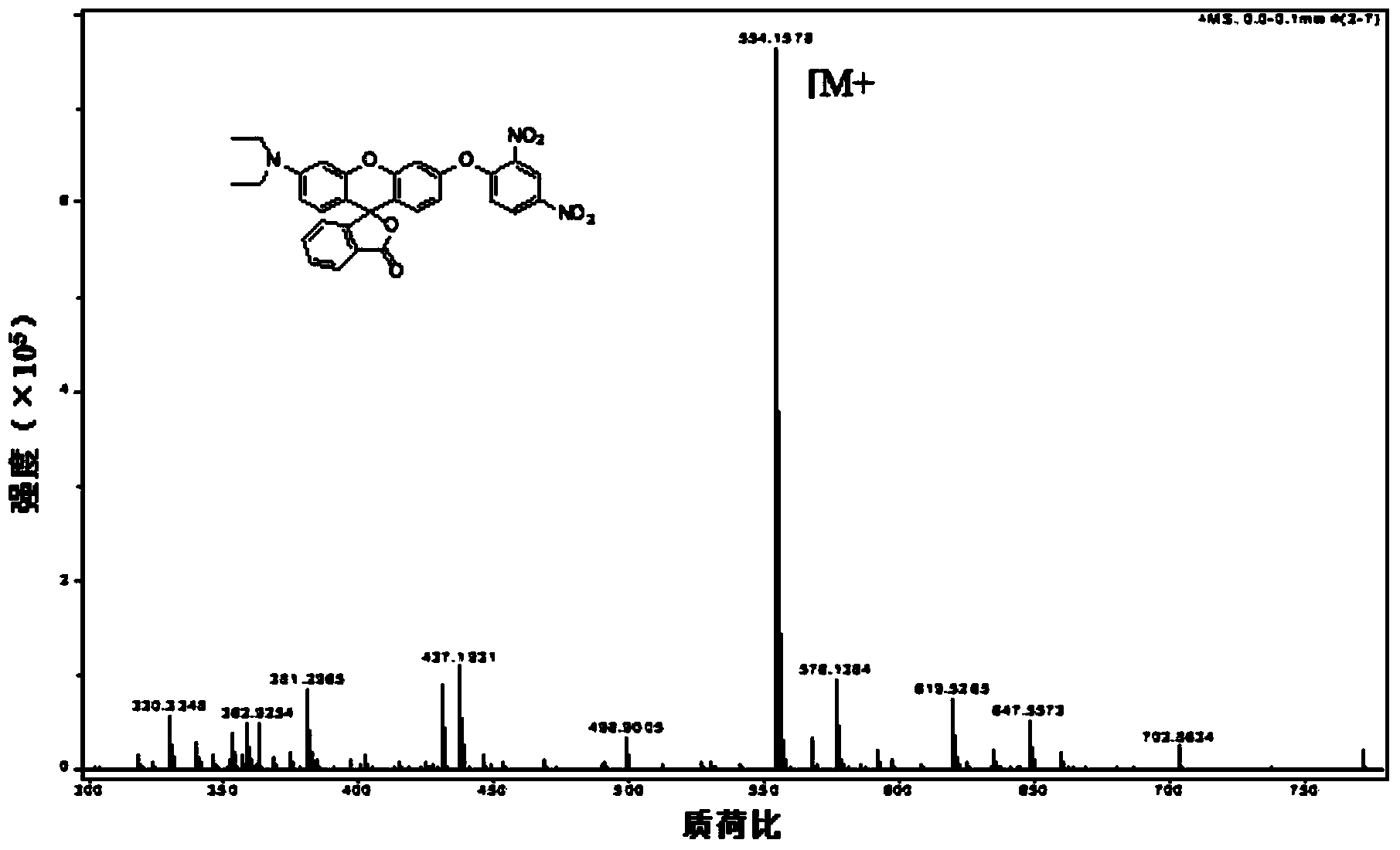
[0018] Synthesis of Fluorescent Probes Containing N,N-Diethyl-p-rhodol
[0019] 1. Synthesis of 3-(N,N-diethyl)-6-hydroxy-fluoran
[0020] Add 2.0g of 3-diethylaminophenol, 1.5g of phthalic anhydride and 15mL of toluene into a 25mL round bottom flask, heat up to 120°C, stir and reflux for 10 hours, cool to 50°C, and use 36% NaOH aqueous solution Adjust the pH value of the reaction system to 11, then raise the temperature to 90°C, stir and reflux for 6 hours, cool and filter, dissolve the filter cake in 50mL distilled water, adjust the pH value to 6 with 20% HCl aqueous solution, filter, and dry , recrystallized with absolute ethanol to obtain the pink product 2'-carboxy-4'-diethylamino-2'-hydroxybenzophenone; add 4.120 mg of 2'-carboxy-4'-di Ethylamino-2'-hydroxybenzophenone, 42mg resorcinol, 3mL methanesulfonic acid, stirred at 90°C for 24 hours, cooled to room temperature, poured the reaction solution into 15mL ice water, filtered, washed with 20mL distilled water, and drie...
Embodiment 2
[0027] The use of the fluorescent probe containing N,N-diethyl-p-rhodol prepared in Example 1 in the detection of thiophenol, its specific detection method is as follows:
[0028] Add 4.0mL absolute ethanol, 2.0mL phosphate buffer solution with a pH value of 7.4, 0.02mL ethyl acetate solution of 1.0mmol / L fluorescent probe, and the sample solution to be tested to a 10mL colorimetric tube, and set Let it stand at room temperature for 25 minutes, and use a fluorescence spectrophotometer to detect the fluorescence intensity of the reaction system at a wavelength of 543nm. According to the formula F=173.65681C+7.89118, calculate the concentration of thiophenol in the sample solution to be tested, where F is the fluorescence intensity of the reaction system at 543nm, and C is the concentration of thiophenol in the reaction system, in μmol / L.
[0029] In order to prove the beneficial effects of the present invention, the inventor has carried out a large amount of laboratory research...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com