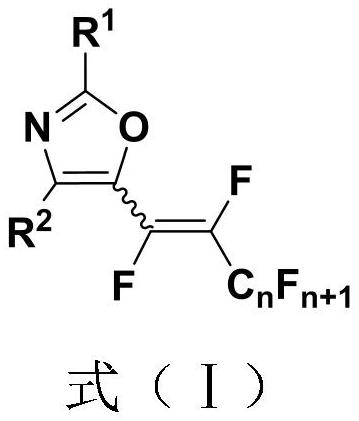
Polyfluoroalkenyl substituted oxazole compound and preparation method thereof
A polyfluoroalkenyl, compound technology, applied in organic chemistry and other directions, to achieve high step economy, mild reaction conditions, diverse functional group tolerance and substrate range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
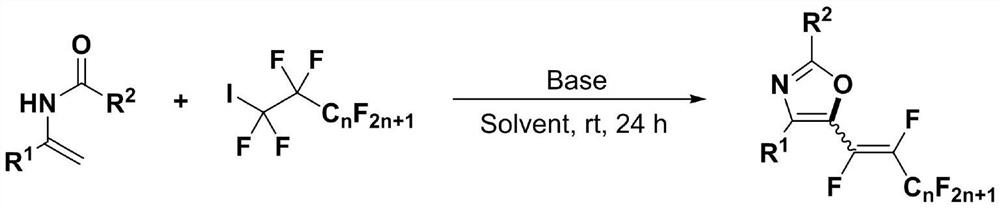
[0037] (1) 1mmol of N-acyl enamine compound (0.162 gram), 2mmol of perfluoroalkane iodide (0.692 gram), 2.5mmol of base accelerator (0.381 gram) were added into a 10mL test tube reaction tube, Add 5mL dimethyl sulfoxide to the reaction tube as a solvent, seal it tightly, and stir and react at room temperature (25° C.) for 24 hours to obtain a polyfluoroalkenyl substituted oxazole compound; wherein, the N-acyl enamine compound is N-( 1-phenylvinyl) acetamide, fluoroiodoalkane is perfluoroiodobutane; base accelerator is diazabicyclic;
[0038] (2) After the reaction in step (1) finishes, the reaction solution is successively dried through water, ethyl acetate, anhydrous sodium sulfate and separated by column chromatography (column chromatography separation condition: the stationary phase is 300~400 mesh silica gel powder, The mobile phase was ethyl acetate (A) and petroleum ether (B), and the mobile phase change program (A:B) was 1:50→1:10, and 0.315 g of reaction product 1 was ...
Embodiment 2~78
[0042] Embodiments 2-78 are basically the same as the above-mentioned embodiment 1, and the difference is as shown in the following table 1:
[0043] Table 1 Examples 2-78
[0044]
[0045]
[0046]
[0047]
Embodiment 79
[0049] (1) Add the reaction raw material formed by mixing 1mmol N-acyl enamine compound and 3mmol perfluoroalkyl iodide into 3mmol base accelerator and 5mL tetrahydrofuran solvent, and stir the reaction under air atmosphere and 90°C temperature for 24 hours, TLC detection determines the reaction process, and the reaction solution is obtained after the end of the reaction; wherein, the N-acyl enamine compound is N-(1-phenylvinyl) acetamide, and the fluoroiodoalkane is perfluoroiodobutane; the base accelerator is a diazabicyclic ring;
[0050] (2) After the reaction in step (1) finishes, the reaction solution is successively dried through water, ethyl acetate, anhydrous sodium sulfate and separated by column chromatography (column chromatography separation condition: the stationary phase is 300~400 mesh silica gel powder, The mobile phase was ethyl acetate (A) and petroleum ether (B), and the mobile phase change program (A:B) was 1:50→1:10 to obtain the reaction product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com