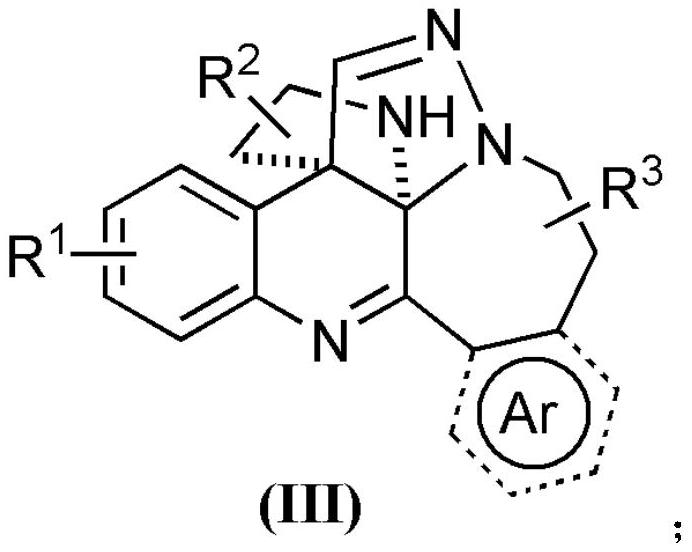
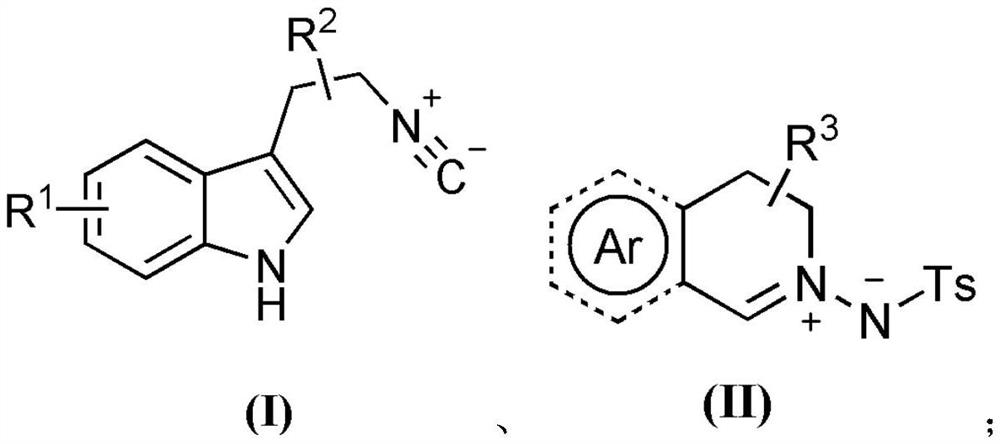
Polycyclic quinoline derivative as well as preparation and application thereof
A technology for polycyclic quinolines and derivatives, applied in the fields of polycyclic quinoline derivatives and their preparation and application, capable of solving the problems of step economy and low efficiency of atom economy, achieving high yield and high atom economy , The effect of cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the compound shown in synthetic (III-1)
[0045] Weigh 0.225mmol tryptamine isocyanide (compound corresponding to number (1), 0.0383g), 0.15mmol C, N imine isoquinoline dipole (compound corresponding to number (12), 0.0450g) in a 20mL test tube In the reaction tube, add 3mL of methanol as a solvent, and stir and react at 25°C for 24 hours; The phases were ethyl acetate (A) and petroleum ether (B), the mobile phase change program (A:B) was 1:6), and 0.0382 g of the reaction product was obtained.
[0046] The above-mentioned reaction product is characterized, and the result is:
[0047] 1 H NMR (400MHz, DMSO- d6 )δ=7.59–7.47(m,3H),7.42–7.24(m,5H),7.00(s,1H),3.82–3.74(m,1H),3.55–3.46(m,1H),3.10–2.95( m,2H),2.76–2.62(m,3H),1.73(td,J=11.6,6.6Hz,1H),one H was overlapped with solvent peak 2.54–2.44;
[0048] According to the characterization data, it can be seen that the obtained reaction product is a pure product (purity>95%); the product yield is calculated,...
Embodiment 2
[0049] Embodiment 2: the compound shown in synthetic (III-2)
[0050] Weigh 0.225mmol 5-methyltryptamine isonitrile (compound corresponding to number (2), 0.0414g), 0.15mmol C, N imine isoquinoline dipole (compound corresponding to number (12), 0.0450g) In a 20mL test tube reaction tube, add 3mL of methanol as a solvent, and stir at 25°C for 24 hours; Silica gel powder, the mobile phase is ethyl acetate (A) and petroleum ether (B), the mobile phase change program (A:B) is 1:6), and 0.0344g of the reaction product is obtained.
[0051] The above-mentioned reaction product is characterized, and the result is:
[0052] 1 H NMR (400MHz, DMSO- d6 )δ=7.52(dd,J=7.4,1.2Hz,1H),7.41–7.33(m,3H),7.32–7.24(m,2H),7.14(dd,J=7.9,1.2Hz,1H),6.97 (s,1H),3.84–3.71(m,1H),3.55–3.42(m,1H),3.12–2.93(m,2H),2.78–2.68(m,1H),2.68–2.58(m,2H) ,2.36(s,3H),1.71(td,J=11.6,6.8Hz,1H),one H was overlapped with solvent peak 2.53–2.43;
[0053] According to the characterization data, it can be seen that the ...
Embodiment 3
[0054] Embodiment 3: the compound shown in synthetic (III-3)
[0055] Weigh 0.225mmol 6-methyltryptamine isonitrile (compound corresponding to number (3), 0.0414g), 0.15mmol C, N imine isoquinoline dipole (compound corresponding to number (12), 0.0450g) In a 20mL test tube reaction tube, add 3mL of methanol as a solvent, and stir at 25°C for 24 hours; Silica gel powder, the mobile phase is ethyl acetate (A) and petroleum ether (B), the mobile phase change program (A:B) is 1:6), and 0.0305g of the reaction product is obtained.
[0056] The above-mentioned reaction product is characterized, and the result is:
[0057] 1 H NMR (400MHz, CDCl 3 )δ=7.65(dd,J=7.3,1.4Hz,1H),7.49(s,1H),7.38–7.29(m,2H),7.23–7.13(m,3H),6.78(s,1H),3.88 –3.80(m,1H),3.68–3.60(m,1H),3.29–3.18(m,1H),3.15–3.05(m,1H),2.88–2.74(m,2H),2.66–2.57(m, 1H), 2.38(s, 3H), 2.02–1.92(m, 1H), 1.74(s, 1H);
[0058] According to the characterization data, it can be seen that the obtained reaction product is a pure prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com