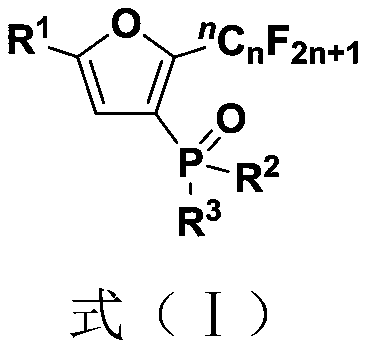
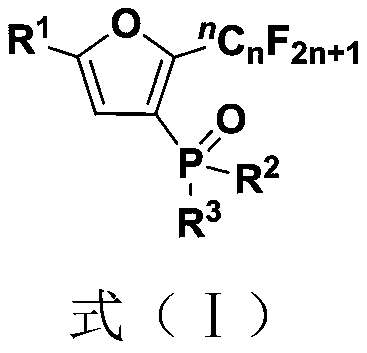
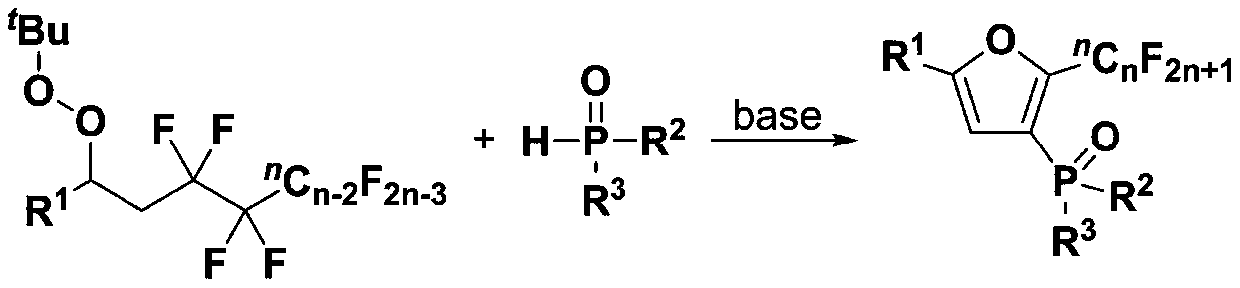
Fluoroalkyl substituted furyl monophosphine oxide compound and preparation method thereof
A technology of furyl mono- and compound, which is applied in the field of fluoroalkyl-substituted furyl monophosphine oxide and its preparation, which can solve the problems of long reaction time of transition metals, limited reaction practicability, and difficulty in realizing phosphine oxidation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) 1 mmol of polyfluoroalkyl substituted peroxy compound (0.4684 gram), 1 mmol of organic phosphine oxide compound (0.2022 gram), 3 mmol of base accelerator (0.9775 gram) were added into a 10 mL test tube reaction tube, Then add 2mL tert-butanol in the reaction tube as a solvent, seal it tightly, and stir and react at room temperature for 24 hours to obtain a fluoroalkyl-substituted furyl monophosphine oxide compound; wherein, the polyfluoroalkyl-substituted peroxy compound is 1-(tert- Butyl peroxy)-2-perfluorobutylethylbenzene, the organic phosphine oxide is diphenylphosphine oxide; the base accelerator is triethylenediamine;
[0040] (2) After the reaction in step (1) finishes, the reaction solution is successively dried through water, ethyl acetate, anhydrous sodium sulfate and separated by column chromatography (column chromatography separation condition: the stationary phase is 300~400 mesh silica gel powder, The mobile phase was ethyl acetate (A) and petroleum et...
Embodiment 2~34
[0044] Embodiments 2 to 34 are basically the same as the above-mentioned embodiment 1, except that in step (1), the polyfluoroalkyl substituted peroxy compound, organic phosphine oxide compound, and alkali accelerator are different, as shown in Table 1 below:
Embodiment 3~34
[0046]
[0047]
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com