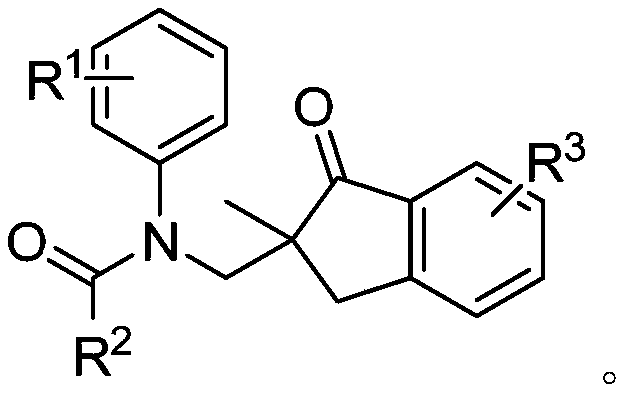
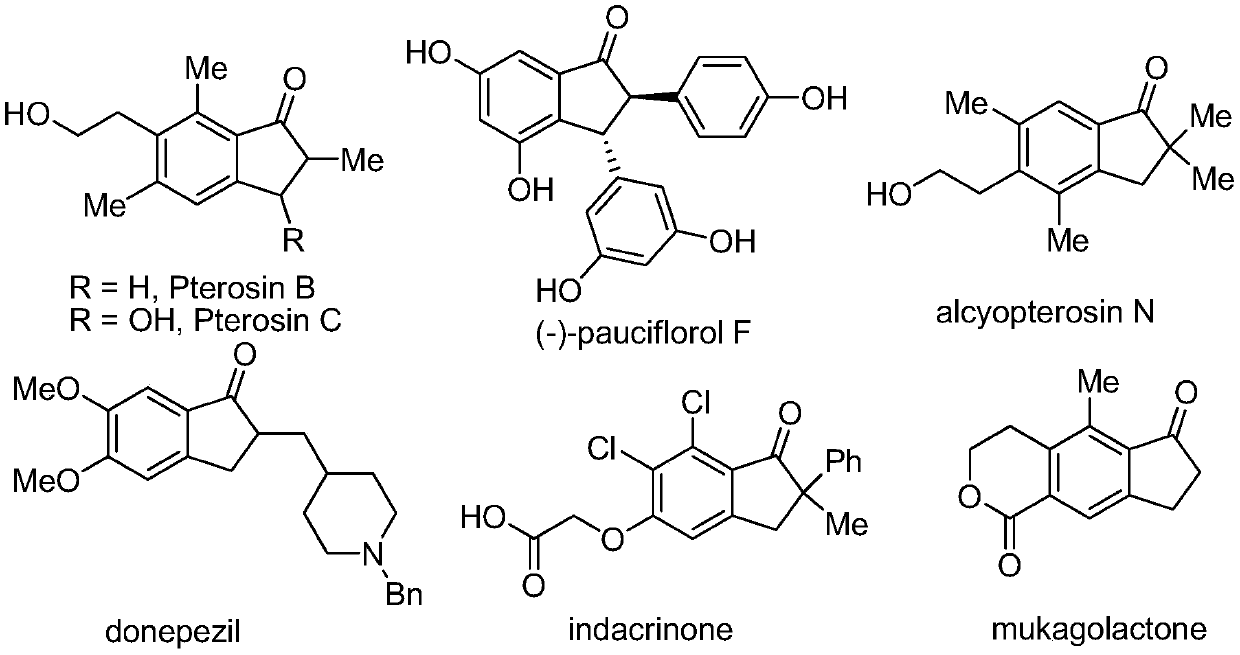
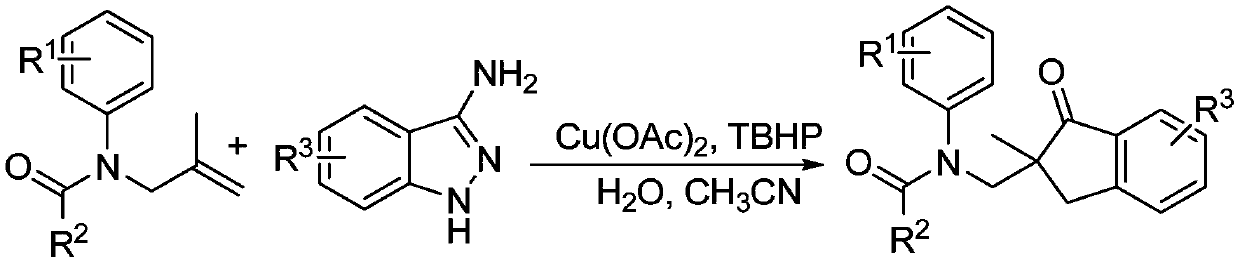Method for synthesizing 2,2-disubstituted indanone compound
A synthesis method and compound technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of cumbersome synthesis methods and low stability of raw materials, and achieve the effect of high economy and high step economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Under nitrogen protection, N-(2-methylpropenyl)-N-phenylacetamide 1a (0.2mmol), 3-aminoindazole 2a (0.3mmol), cuprous iodide (20mol%), peroxide Tert-butanol oxide (TBHP, 70% aqueous solution, 0.4 mmol), water (0.4 mmol), acetonitrile (1.5 mL), was added to a Schlenk reaction tube and sealed. Heating to 80°C, the reaction time was 10 hours. After the reaction, the solvent was removed under reduced pressure, and the target product 3aa was obtained by column chromatography with a yield of 72%. 1 H NMR (400MHz, CDCl 3 ): δ7.58(t, J=7.3Hz, 1H), 7.45(t, J=7.9Hz, 2H), 7.30(t, J=7.4Hz, 1H), 7.14-7.22(m, 3H), 6.71 (s,2H),4.58(d,J=13.8Hz,1H),3.69(d,J=13.8Hz,1H),3.41(d,J=17.8Hz,1H),2.86,(d,J=17.8 Hz,1H),1.75(s,3H),1.14(s,3H). 13 C NMR (100MHz, CDCl 3 ): δ208.8, 171.5, 152.6, 142.6, 135.8, 134.9, 129.1, 127.8, 127.2, 126.5, 124.0, 54.2, 50.0, 37.6, 23.6, 22.7.
Embodiment 2
[0029] Under nitrogen protection, N-(2-methylpropenyl)-N-p-tolylacetamide 1b (0.2mmol), 3-aminoindazole 2a (0.3mmol), copper acetate (10mol%), peroxide Tert-butanol (TBHP, 70% in water, 0.5 mmol), water (0.6 mmol), acetonitrile (2.5 mL), was added to a Schlenk reaction tube and sealed. Heating to 80°C, the reaction time was 10 hours. After the reaction, the solvent was removed under reduced pressure, and the target product 3ba was obtained by column chromatography with a yield of 81%. 1 H NMR (400MHz, CDCl 3 ): δ7.56(t, J=7.4Hz, 1H), 7.45(q, J=7.6Hz, 2H), 7.29(t, J=7.3Hz, 1H), 6.93(d, J=7.5Hz, 2H ),6.57(s,2H),4.55(d,J=13.8Hz,1H),3.65(d,J=13.8Hz,1H),3.40(d,J=17.8Hz,1H),2.84,(d, J=17.8Hz,1H),2.26(s,3H),1.72(s,3H),1.13(s,3H). 13 C NMR (100MHz, CDCl 3 ): δ208.9, 171.6, 152.6, 140.0, 137.5, 135.8, 134.8, 129.7, 127.4, 127.1, 126.5, 124.0, 54.2, 49.9, 37.6, 23.6, 22.6, 20.9.
Embodiment 3
[0031]Under nitrogen protection, N-(2-methylpropenyl)-N-m-tolylacetamide 1c (0.2mmol), 3-aminoindazole 2a (0.4mmol), copper trifluoromethanesulfonate (10mol%) ), hydrogen peroxide (30% aqueous solution, 0.5 mmol), water (0.4 mmol), and acetonitrile (1.5 mL), were added into the Schlenk reaction tube and sealed. Heating to 80°C, the reaction time was 10 hours. After the reaction, the solvent was removed under reduced pressure, and the target product 3ca was obtained by column chromatography with a yield of 63%. 1 H NMR (300MHz, CDCl 3 ):δ7.55-7.60(m,1H),7.45(d,J=6.6Hz,2H),7.29(t,J=7.4Hz,1H),6.96-7.07(m,2H),6.56(s, 1H),6.34(s,1H),4.57(d,J=13.8Hz,1H),3.66(d,J=13.8Hz,1H),3.39(d,J=17.8Hz,1H),2.84,(d ,J=17.8Hz,1H),2.07(s,3H),1.73(s,3H),1.13(s,3H). 13 C NMR (75MHz, CDCl 3 ): δ208.7, 171.4, 152.6, 142.3, 139.0, 136.0, 134.7, 128.9, 128.6, 128.5, 127.1, 126.5, 124.6, 123.8, 54.2, 49.8, 37.4, 23.6, 22.6, 21.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com