Polysubstituted benzothienopyridine compound and preparation method thereof
A technology of phenopyridine and benzothiopyridine, which is applied in the field of multi-substituted thienopyridine compounds and their preparation, can solve the problems of reaction research limitations and achieve the effects of high atom economy, high step economy and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
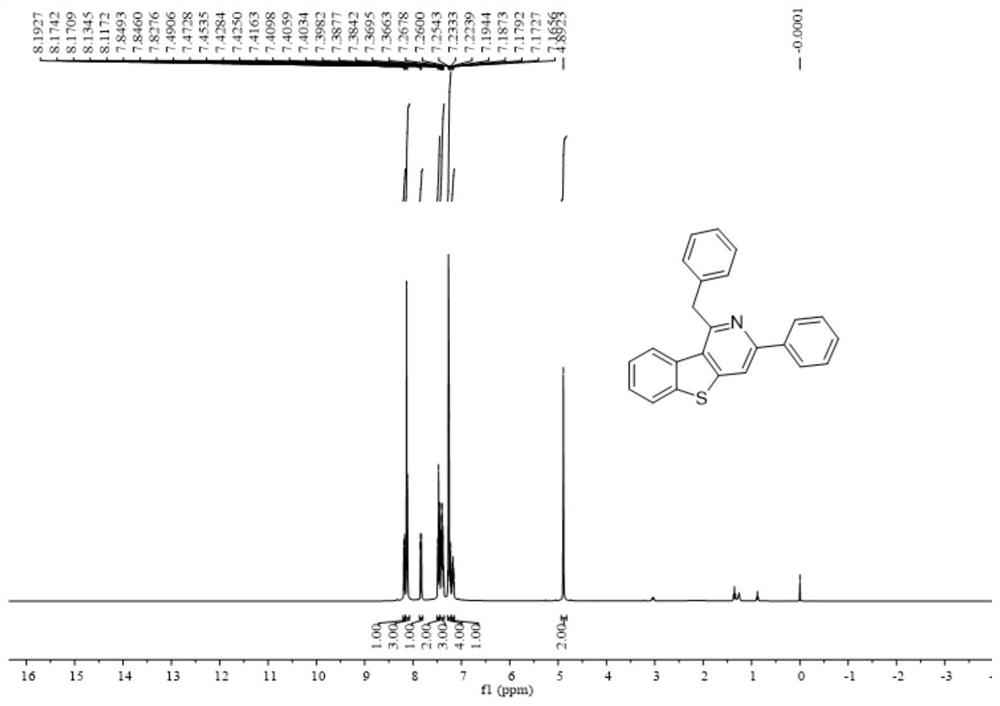
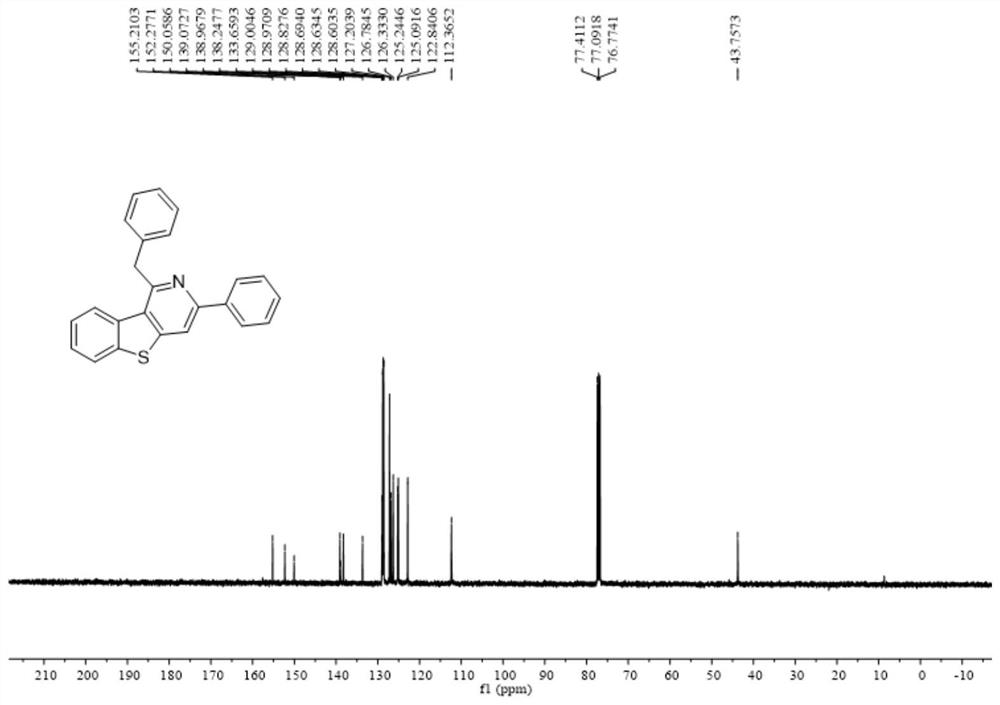
[0044] Add 0.2 mmol 2-methyl-3-(phenylethynyl) benzo[b]thiophene, 0.6 mmol benzonitrile, 0.4 mmol bis(trimethylsilyl) lithium amide, 0.24 Millimoles of potassium tert-butoxide and 0.5 ml of anhydrous cyclopentyl methyl ether solvent were stirred and reacted at 120 degrees Celsius at 500 rpm for 24 hours, and the stirring was stopped. Add 5 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous sodium sulfate to dry, filter, concentrate under reduced pressure, and then separate and purify by column chromatography to obtain the target product, which is eluted by column chromatography The liquid is petroleum ether:ethyl acetate mixed solvent with a volume ratio of 100:1, and the yield is 90%.
[0045] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows figure 1 and figure 2 shown; the structural characterization data are shown below:
[0046] 1 H NMR (400MHz...
Embodiment 2
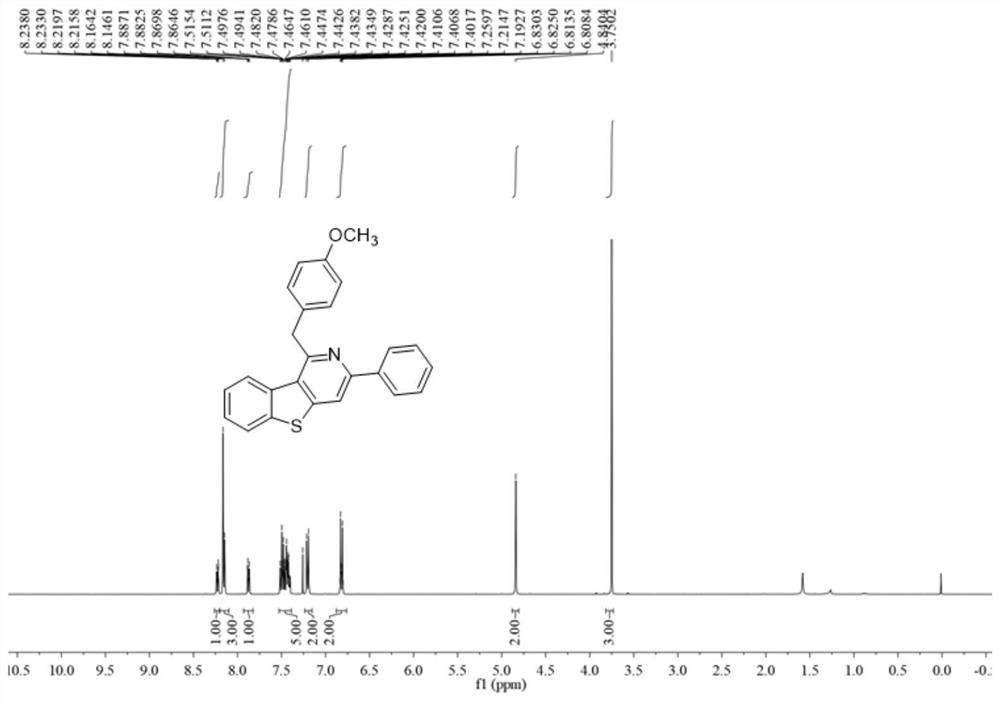
[0053] Add 0.2 mmol 3-((4-methoxyphenyl)ethynyl)-2-methylbenzo[b]thiophene, 0.6 mmol benzonitrile, 0.4 mmol bis(trimethyl Silicon-based) lithium amide, 0.24 mmol of potassium tert-butoxide and 0.5 ml of anhydrous cyclopentyl methyl ether solvent were stirred and reacted at 120 degrees Celsius at 500 rpm for 24 hours, and the stirring was stopped. Add 5 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous sodium sulfate to dry, filter, concentrate under reduced pressure, and then separate and purify by column chromatography to obtain the target product, which is eluted by column chromatography The liquid is petroleum ether with a volume ratio of 40:1: ethyl acetate mixed solvent, and the yield is 71%.
[0054] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows image 3 and Figure 4 shown; the structural characterization data are shown below:
[0055] 1 ...
Embodiment 3
[0062] Add 0.2 mmol N,N-dimethyl-4-((2-methylbenzo[b]thiophen-3-yl)ethynyl)aniline to the reaction tube, 0.6 mmol benzonitrile, 0.4 mmol Lithium bis(trimethylsilyl)amide, 0.24 mmol potassium tert-butoxide and 0.5 ml anhydrous cyclopentyl methyl ether solvent were stirred and reacted at 120 degrees Celsius at 500 rpm for 24 hours, and the stirring was stopped. Add 5 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous sodium sulfate to dry, filter, concentrate under reduced pressure, and then separate and purify by column chromatography to obtain the target product, which is eluted by column chromatography The liquid is sherwood oil with a volume ratio of 10:1: ethyl acetate mixed solvent, and the yield is 83%.
[0063] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows Figure 5 and Figure 6 shown; the structural characterization data are shown below:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com